Introduction
Due to the raise of international trade, the number of exotic plants has been increasing in South Korea (Ko et al., 2019; Oh et al., 2003; Park et al., 2020). Available data suggest that approximately 321 species of exotic plants have adapted to South Korea, and approximately 100 of these exotic weeds have inflowed since 2009 (Park, 2009). The origin of those plants was followed by Europe (134 species, 42%), North America (75 species, 23%), Eurasia (28 species, 9%) and tropical America (27 species, 8%). These naturalized plants expand in habitat and ecosystem, threatening native flora and disturbing ecosystems (Kim et al., 2000; Ko et al., 2019).
Among naturalized plants, Chenopodium album is a fast-growing weedy annual plant in the genus Chenopodium, originated from Europe (Bassett and Crompton, 1978). It is known by many common names as fat hen, white goosefoot, and common lamb’s quarters weeds. It occurs as a common weed in agricultural areas and habitation in temperate and sub-tropical regions. It thrives on all soil types and over a wide range of pH values (Coleman et al., 2020; Holm et al., 1977). C. album usually prefer abundant in sunlight, moisture levels, and fertile loamy soil for growth and development. C. album is one of the most widely distributed species of exotic weeds over the world and is one of the most successful colonizers as it moves into new areas. It is one of the most serious weed in 15 countries (Holm et al., 1979) and a predominant weed in 34 additional countries in all kinds of cultivated land (Uotila, 2001).
C. album was initially recorded in Korea before 1949 and it was distributed in Gyeonggi, Gangwon, Chungbuk, Chungnam, Jeonbuk, Jeonnam, Gyeongbuk, Gyeongnam, Seoul, Incheon, Busan, Ulsan, Daegu, and Jeju (Jung et al., 2017). Up to now, Huh (2019) has reported the distribution of C. album in Korea, where it occurred in middle and low regions except upper region. It is also found on wasteland, in pastures, strips of uncultivated land, and along roadsides and riverbanks (Hwang et al., 2017). Won et al. (2011) reported that a field having C. album with a density of 192 plants m-2 decreased the predicted dried pepper yield by approximately 54 times when compared with a pepper field without C. album. Hwang et al. (2015) indicated C. album is the most dominant species, found in onion and Chinese cabbage fields at Chungnam region in 2014, become problematic in horticulture fields. Lee et al. (2017) evaluated C. album and found it among top ten the most dominant exotic weeds in arable land in Korea.
Despite the increasing problem of C. album in farming systems of Korea, there is a few published information on the seed germination and seedling establishment of C. album. To optimize weed control and maximize the efficiency of management tactics, biological and ecological information, specifically germination requirements, should be examined. Therefore, the objectives of this study were to determine the effect of different environmental factors on the seed germination and seedling emergence of C. album.
Materials and Methods
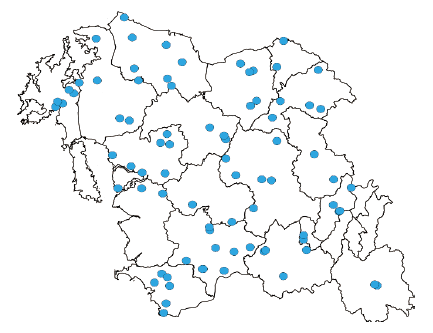
Distribution of Chenopodium album in Chungnam province of Korea
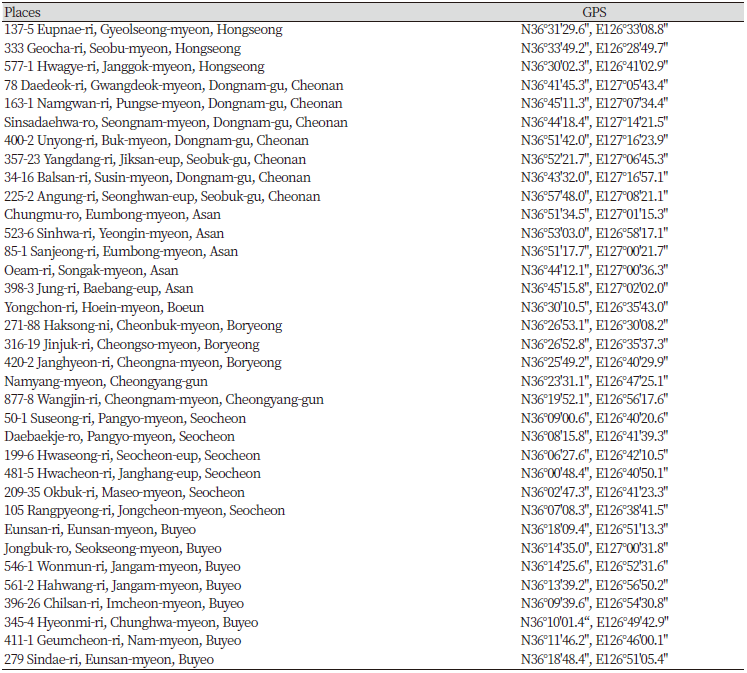
This study was conducted to investigate the distribution of C. album at 132 sites in 18 cities of Chungnam province in Korea. The distribution characteristics of C. album and regions were analyzed based on the survey data and the spatial and regional distributions were analyzed using the recorded GPS data. A distribution map of C. album was prepared using QGIS (v2.18.13, https://www.qgis.org).
Dormancy of C. album seeds after harvest
The mature seeds of Chenopodium album were collected in November 2018 at Chungnam National University (N36°22'09.6". E127°21'14.1"). Heteromorphic seeds were stored at room temperature (20-25℃), and retrieved monthly for seed germination tests, beginning on November 2018 (after harvest) at the end of November 2019. Twenty seeds were incubated on two layers of filter paper in a Petri dish (60×15 mm), to which 5 mL of distilled water. The dishes were sealed with a parafilm and placed in a germinator set at 16/8 h light/dark. Final germination was determined seven days after sowing (DAS). Non-germinated seeds were checked under a stereomicroscope to see if the embryos were white and firm, thus indicating they were alive, otherwise they were considered dead.
Germination by temperature
Three replicates of seeds from each seed type (black and brown seeds) were tested in each treatment. For germination, twenty seeds were incubated on two layers of filter paper in a Petri dish (60×15 mm), to which 5 mL of distilled water. The dishes were sealed with a parafilm and placed in a germinator set at 15/5, 20/10, 25/15, 30/20, 35/25℃ (day/night), 16/8 h light/dark condition. Final germination was determined at 7 DAS.
Investigation of salt stress
To investigate the effect of salinity on the germination of heteromorphic seeds, different concentrations of NaCl solution (0, 10, 20, 40, 80, 160, and 320 mM) were used in the germination test. Twenty seeds were incubated on two layers of filter paper in a Petri dish (60×15 mm), to which 5 mL of NaCl were added. For each of these trials, the treatment with sterilized distilled water was included as a control. All petri dishes were placed in an illuminated incubator subjected to a 16h daily photoperiod at 20-25/10-15℃ (day/night). The seed germination was determined at 7 DAS.
Emergence by burial depth
Ten brown seeds were sown into a plastic pot (size: 7 cm diameter, height 10 cm) filled with mixed soil (1:1 [w/w]) of horticultural nursery soil:rice nursery soil. Burial depths were 0.5, 1, 2, 3, and 4 cm and replications were three. At 21 DAS, the number of seedlings was counted. Plastic pots were placed in a greenhouse subjected to a 16h daily photoperiod at 20-25/10-15℃ (day/night).
Total seed protein content analysis
To gain insight into the total protein variability that may correlate with seed heteromorphism in C. album, the total seed protein was measured. Black and brown seeds (100 mg of each) were homogenized in liquid nitrogen and then transferred to a fresh 1.5 mL micro-tube with 500 µL extraction buffer (12.5 mmol L-1 TrisHCl, pH 6.8). The homogenate was centrifuged at 10,000 g for 15 min at 4℃. The resulting supernatant was stored at 4℃ until use. The protein content of the extract was determined by using Bradford method (1976).
Statistical analyses
All data in this study were analyzed using the Statistical Analysis System software (SAS version 9.2, SAS Institute Inc., Cary, NC, USA) and expressed as mean±standard deviation (SD) of three biological replicates. The significant differences among the means were calculated using analysis of variance (ANOVA) with Duncan’s multiple range test (DMRT).
Results and Discussion
Distribution of C. album in Chungnam province of Korea
According to survey data, there were 85 sites where C. album plants were found. C. album distributed along the roadsides (18%), wastelands (4%), near animal farms (25%) and greatly in crop fields (43%). It can be expressed as contamination of the seeds is a plausible reason for C. album dispersion. This phenomenon will be more serious and unexpectable if not be controlled. Therefore, regular monitoring of the C. album in agro-ecosystem could be suggested as a basic precaution to prevent the invasiveness of C. album in the future (Fig. 1; Supplemental data).
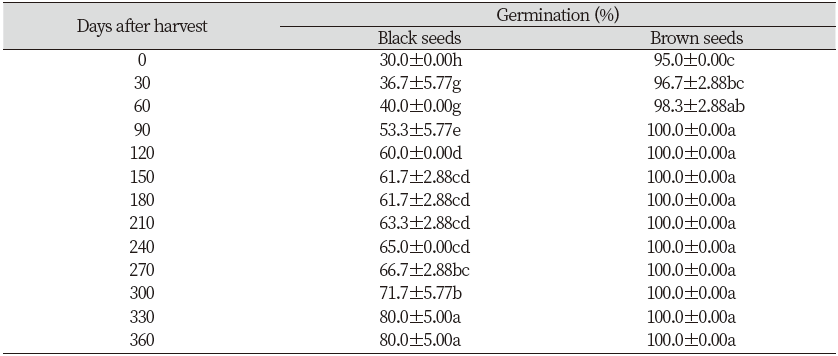
Dormancy of C. album seeds after harvest
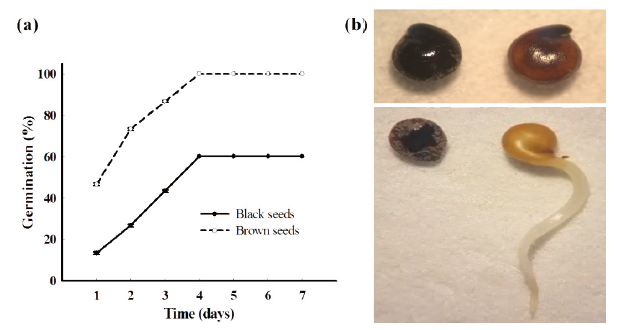
Considering the pooled data from two types of C. album seed, dry storage at room temperature had little effect on the germination of the brown seeds. The freshly mature brown seeds were non-dormant for the whole year. In contrast, there was considerable variation in the dormancy behavior of the black seeds. At the beginning of the experiment, only 30% of the black seeds germinated while nearly 100% germination was observed during most of the year (Table 1). The dormancy of black seeds can be alleviated by keeping them at room temperature. Approximately 80% of black seeds germinated at 330 days after storage (Table 1). Besides, the germination rate of the heteromorphic seeds differed dramatically. The brown seeds germinated faster and seed germination rate was higher than the black seeds. (Table 1; Fig. 2a and 2b).
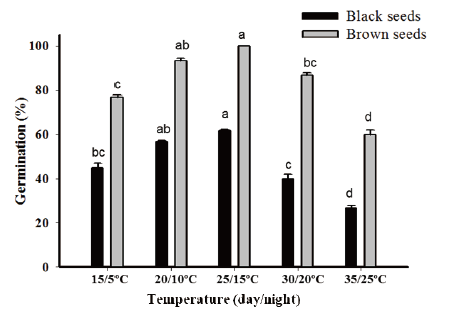
Germination by temperature
C. album germinated in a wide range of temperatures in a growth chamber. The highest growth rate was observed at 20/10℃ to 25/15℃ (day/night), and a temperature above or below the optimum growth temperature corresponded to a decrease in growth (Fig. 3).
Bhowmik (1982) mentioned that germination of C. album starts in late autumn and continues to mid-spring, grew best at 25℃. Moreover, C. album is reported as C3 plant, and plants that survive solely on C3 fixation tend to thrive in areas where temperatures are moderate 20 to 25℃ (Wataru et al., 2014). To explain more in detail, we also refer to the difference between the day and night temperature. According to Ann (2008) plants grow best when the daytime temperature is about 10 to 15 degrees higher than night time temperature. Our results were also similar to this report. An optimum temperature was 20/10℃ to 25/15℃ (day/night) for the germination of both type seeds.
Temperature played an important role in germination, as temperature higher or lower than optimum temperature; plants often shows low germination rate and poor growth (Ann, 2008). Our results are compatible with above conclusion. The germination of C. album was reduced at both high temperature (30, 35℃) and low temperature (15℃).
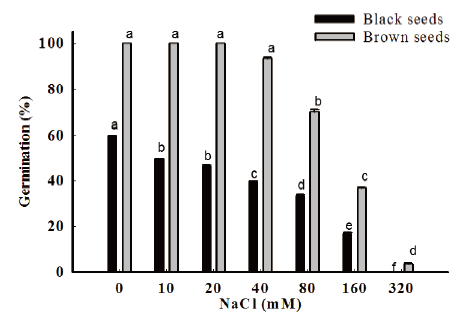
Investigation of salt stress
The effect of salinity on the growth of the plants derived from black and brown seeds was different. Under various salinity, the germination rate of black seeds was lower than brown seeds. C. album acclimatized to a salinity of 80 mM or less, grow well in a range of salinities 10 to 40 mM. At salinity of 160 mM, the percentage of germination decreased, and it was significantly inhibited by higher salinities (Fig. 4). Yet, 10% brown seeds could survive at the highest salinities of 320 mM while no black seeds germinated.
Yao et al. (2010) reported that C. album is a salt-tolerant species having the ability to germinate and grow successfully under saline conditions. In China, more than 50% seeds of C. album were brown colored and germinated normally under a very high sodium chloride concentration of 300 mM (Yao et al., 2010).
Our results suggest in sites where the soil salinity is ≤160 mM NaCl, seeds of C. album can germinate successfully. However, in sites where soil salinity exceeds 160 mM NaCl, seeds do not germinate or low germination might be occured. Thus, they may remain in the soil seed bank until the salt is diluted by water from melting snow or rainwater and proper environmental factor will sprout the seeds. As such, soil salinity would play an important ecological role in the establishment of C. album populations and successful establishment helps this species to infest diverse cropping systems and become invasive in the future.
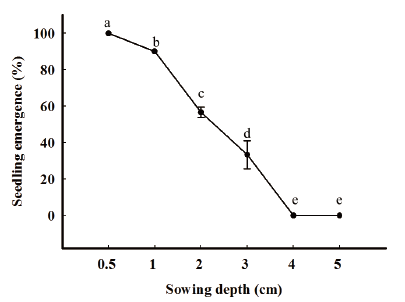
Emergence by burial depth
The effect of sowing depth on seedling (brown seeds) emergence was significant. Seedling emergence decreased markedly in parallel with increasing seed sowing depth. The highest rates of seedling emergence were observed at 0.5 cm of seeding depth, a large percentage of the seeds (approximately 90%) emerged at 1 cm burial depth. There was a significant decline in seedling emergence at 2 and 3 cm burial depths. At 2 cm of burial depth, 50% seeds and only 33% of seeds emerged at depth of 3 cm, whereas no seedlings emerged at sowing depth 4 cm and deeper (Fig. 5).
Our results are in line with other research on the effect of burial depth on seedling emergence. The seedling emergence declined dramatically with increasing the burial depth (Bond et al., 1999; Grundy et al., 2003). This result could be explained as follows; C. album belongs to small seed group when we increase sowing depth the seedling emergence decrease was higher compare with large seeded species. Moreover, at deep burial depths, the seed mortality also was higher than large seeded species. Lahoreau et al. (2006) reported that small-seeded species are subject to selection pressure to adaptation as light, soil, and fluctuating temperatures in germination experiments.
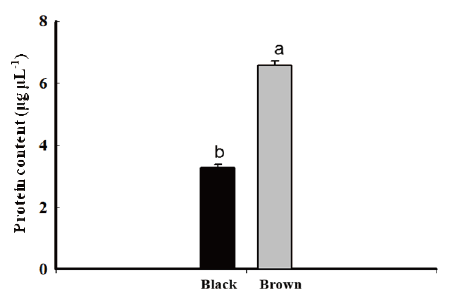
Total seed protein content analysis
The total seed protein content was much lower in the black seeds (3.5 μg μL-1) than in the brown seeds (3.5 μg μL-1). That is more energy might be available for radicle to break the seed coat in the brown seeds, which contributes difference in the germination for the black seeds (Fig. 6).
Schweizer and Ries (1969) and Ries et al. (1970) reported a positive relationship between plant growth and the protein content of the seed planted. In seed that contained high levels of protein, water imbibition and oxygen consumption were accelerated, the speed of germination (Mayer and Poljakoff-Mayber, 1963).
Conclusion
C. album produces two kinds of seeds, which are black and brown seeds. The freshly mature brown seeds were non-dormant for the whole year. In contrast, black seeds exhibit deep dormancy. An optimal temperature for germination of both black and brown C. album seeds was 20/10℃ to 25/15℃ (day/night). At the highest salinities (320 mM), a small proportion (10%) of brown seeds germinated while no black seeds germinated. The total seed protein content was much lower in the black seeds than in the brown seeds suggested that more energy might be available for radicle to break the seed coat in the brown seeds. The seeds full emerged at 0.5 cm and no emergence was seen beyond 4 cm burial depth. Our results on the effects of environmental factors on the growth of C. album could be useful to maximize the efficiency of management tactics in the C. album infested area in Korea.
Acknowledgements
This work was carried out with the support of the “Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ01321601)” Rural Development Administration, Republic of Korea.
Authors Information
Thi Hien Le, Chungnam National University, Ph.D. student
Seung Chul Shin, Chungnam National University, Ph.D. student
Jae Eun Song, Agriculture Research Center, Doctor of Philosophy
Aung Bo Bo, Chungnam National University, Ph.D. student
Weiqiang Jia, Chungnam National University, Doctor of Philosophy
Kwang Min Cho, Daeseung Bio Farm Research Center, Doctor of Philosophy
Kee Woong Park, Chungnam National University, Professor