Introduction
The intensification of trade, transport, and travel is leading to increased introduction of many exotic weeds worldwide (Meyerson and Mooney, 2007). Exotic weeds are referred to by several names, which are often used interchangeably: introduced, non-indigenous, alien or foreign species. Exotic weeds can be defined by the following phenomenon of United State Department of Agriculture (USDA) as “Not native to the continent on which it is now found” (Dyer, 2015). Exotic weeds present a threat to the economic productivity and ecological integrity of natural systems because of fast growth rate, quick regenerative and reproductive potential (Mack et al., 2000; Pimentel et al., 2005). Thus, the accidental or intentional introduction of exotic weeds continues to threaten natural and agricultural ecosystems worldwide (Mack et al., 2000; Pimentel, 2011). As an important trade nation, the exotic weeds inflow into Korea has increased (Hwang et al., 2017a; Oh et al., 2004) and Korea is facing problems with exotic species, which may become invasive in the future.
Chenopodium album L. is primarily known as a widely distributed and highly polymorphic species. C. album originated from Europe, belonging the genus Chenopodium, family Chenopodiaceae and the species name Chenopodium album L. which is derived from the particular shape of the leaf, looking similarly to a goose's foot (Wikipedia, 2018). The botanical classifications of C. album are as follows: Kingdom (Plantae), Class (Magnoliopsida), Family (Chenopodiaceae), Genus (Chenopodium L.), Species (Chenopodium album L.) (USDA, 2018). It occurs as a common winter weed in agricultural areas and habitation in temperate and sub-tropical regions, but it also invades natural vegetation. It is one of the most serious weed in 15 countries (Holm et al., 1979) and a predominant weed in 34 additional countries (Rice, 1984) and nowadays it is a common weed in almost 40 croplands in 47 countries (CABI, 2018) and so widely distributed in both the northern and southern hemispheres, particularly, in Asia, North America, Europe (Brenan and Akeroyd, 1993), India, South Africa, Australia, South America (Williams, 1963) and North America (Bassett and Crompton, 1978; Lorenzi and Jeffery, 1987). It is an extremely common weed in all kinds of cultivated land (Uotila, 2001).
Morphology and biology
Stems
Stems are erect, branched towards apex (Plantwise Knowledge Bank, 2018), and variably colored, ranging from light blue-green to striped with purple and green (Williams, 1963). Young stems are covered with a fine mealy pubescence, while older stems become more glabrous.
Leaves
Leaves are between 3-6 cm in length, 2-4 cm wide, moderately to densely mealy (Pacific island ecosystems at risk, 2018), blade-margins sparsely toothed (Nature gate, 2018). They vary in color but are often green above, and mealy-white below, and may have a mealy (powdery) surface. Lower leaves are triangular with wedge-shaped base; middle leaves are rhomboid or oval-rhomboid, unequally dentate along the edge. Upper leaves are lanceolate, entire (AgroAtlas, 2018).
Flowers
Flowers are green and white, in densely clustered panicles and continuous along the stem (Coleman et al., 2018). Flower perfect, small, calyx of 5 sepals that are more or less keeled and nearly covering the mature fruit, petals 1, stamens 5, pistil 1, with 2 or 3 styles, ovary single-celled, attached at right angles to the flower axis. Flowers hermaphrodite, inconspicuous, odourless and wind-pollinated (Williams, 1963). C. album blooms from June to September (Ketevan et al., 2016). C. album flowers in any day-length, but an 8-hour photoperiod considerably hastens flowering and maturity. Larger, more vigorous plants may result from a long photoperiod (16 to 18 hours), and for this reason the species is more extensively distributed in temperate zones and sparsely distributed around the equator (Holm et al., 1977).
Seeds
Seeds are horizontally flattened, lenticular, averaging 1.1 mm to 1.5 mm in diameter (Wagner et al., 1999), weight 1.2 mg (Plantwise Knowledge Bank, 2018). Enclosed by membranous pericarp, which is easily detached. C. album reproduces solely by seed. C. album produces two distinct lots of seeds (morphs), some (approx. 3.0%) with a brown seed coat (approximately 5% of the seeds) are non-dormant and germinate rapidly after the initial harvest, the majority with a black seed coat (95%), are dormant (Williams and Harper, 1965; Coleman et al., 2018).
Distribution and habitat
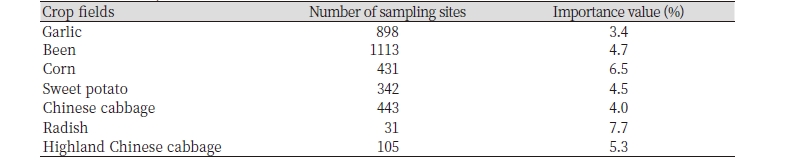
Chenopodium album L. is annual weed (Bassett and Crompton, 1978) and it was initially recorded in Korea before 1949 and it were distributed in Gyeonggi, Gangwon, Chungbuk, Chungnam, Jeonbuk, Jeonnam, Gyeongbuk, Gyeongnam, Seoul, Incheon, Busan, Ulsan, Daegu, and Jeju (Jung et al., 2017). The frequency of C. album occurred in the upland crop fields in Korea 2014 was shown in Table 1. Up to now, Huh (2019) has reported occurrence of C. album in Korea, which is middle and low regions and except upper region. It is also found on wasteland, in pastures, strips of uncultivated land, and along roadsides and riverbanks (CABI, 2018; Hwang et al., 2017b). As described above C. album is one of the most widely distributed species of weeds in the world and is one of the most successful colonizers as it moves into new areas. It thrives on all soil types and over a wide range of pH values (Holm et al., 1977). In general, it prefers temperate to sub-tropical conditions, with higher rainfall (Coleman et al., 2018). C. album usually prefer abundant in sunlight, moisture levels, and a fertile loamy soil for growth and development.
Economic loss
Exotic species may cause ecological, economical, especially in the case when they become invasive. Until now, C. album is one of the more robust and competitive weeds, capable of producing crop losses in corn, soybeans, and sugar beets (Ketevan et al., 2016). At the density of 172 to 300 plants/m², C. album was reported to cause between 6 and 58% yield loss in maize in field experiments in Canada (Ngouajio et al., 1999; Sibuga and Bandeen, 1980). In Spain, Torner et al. (1995) reported 22.3% maize yield loss in irrigated field experiments when maize was allowed to compete with C. album at equivalent densities. 59% maize yield losses were attributed to uncontrolled populations of C. album in field experiments in the USA (Dyck and Liebman, 1995). In North Carolina, USA, Shurtleff and Coble (1985) observed 15% loss of soybean seed yield at a density of 1.6 C. album plants/m of soybean row. In Iowa, USA, Staniforth and Lovely (1964) observed about 35% soybean yield losses due to a natural weed population composed mainly of C. album. In field studies conducted in Colorado, USA, yield of sugar beet decreased as the density of common C. album increased. At densities of 6, 12, 18, and 24 common C. album plants/30 m of row, root yields were reduced 13, 29, 38, and 48%, respectively (Schweizer, 1983). When grown with a high density of 170 C. album plants/m², sugar beet root yield was reduced by 86% (Holm et al., 1977).
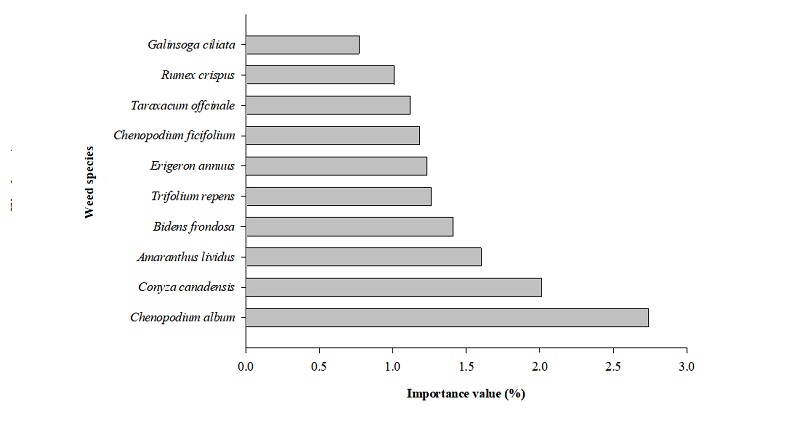
In Korea, Won et al. (2011) reported that field having C. album with density 192 plants/m2 decreased the predicted dried pepper yield by approximately 54 times when compared with pepper field without C. album. Hwang et al. (2015) indicated C. album is the most dominant species, which were found in onion and chinese cabbage fields at Chungnam region in 2014, become problematic in horticulture fields. Lee et al. (2017) evaluated C. album and found it among top ten of most dominant exotic weeds in arable land in Korea (Fig. 1).
While yield losses due to C. album vary according to crop, weed density and location, in all cases reported, the crop losses were of significant economic impact.
Management strategies
According to the article 8(h) (CBD, 1992) in Convention on Biological Diversity (CBD) identified obliges parties to “Prevent the introduction of, control or eradicate those alien species which threaten ecosystems, habitats and species”. To manage these noxious weeds, a number of strategies have been tested such as physical control, chemical control biological control.
Physical control
Populations of weed can be reduced by effective integrated management involving varieties, crop rotation, changing spatial arrangement nutrient management and cropping date (CABI, 2018). Late seeding in particular can give some control because C. album tends to germinate early in the season and those seedlings are killed through soil preparation (BugwoodWiki, 2016). In areas where the population density of C. album is very high, using stale seedbed technique can be applied to germinate weed seeds that disturbs and bring them to the soil surface during cultivation, so that the young weeds can then be eliminated. Moreover, incorporating of winter cover crops disrupts C. album life cycle and greatly inhibits emergence in spring. Control measures are cleaning of crop seeds, crop rotations, careful plowing. In tilled crops, timely inter-row tillage, in uncultivated land, mowing of plants before flowering time. Mechanical weed control operations, such as rotary hoeing and row cultivating can effectively control seedling C. album (BugwoodWiki, 2016).
Chemical control
In fact, weeds quickly regenerate from the plant organs in the soil and produce seedlings that cover the ground lead to high competition with crops. Toole and Brown, (1946) reported the seeds of C. album can remain viable in the soil up to 40 years (Illinois Wildflowers, 2017; Williams, 1963) and seeds remain viable even if submerged in water up to half a year (AgroAtlas, 2018). Once the plant matures and starts producing seeds, physical removal at this stage only help temporary disappearance of C. album because they will soon emerge from seeds in the soil. Thus, the best method of control is before they can form seeds.
Since 1950, herbicide use has increased dramatically; the new herbicides rapidly resulted in revolutionary changes in weed control strategies in industrialized countries (Bo et al., 2017). Up to now, numerous synthetic herbicides have been tried for the management of C. album. DiTomaso et al. (2013) reported using chemical herbicide such as 2,4-D, aminopyralid, chlorsulfuron, dicamba, glyphosate, hexazinone, imazapic, imazapyr, metsulfuron, paraquat, picloram, sulfosulfuron, triclopyr have provided excellent control, generally better than 95%. Rimsulfuron provided good control results (80-95%) of C. album.
Lorenzi (1984) and Mamarot and Rodriguez (1997) provide suggestions for using herbicides treatment in a wide range of crops in Brazil and France. Using isoproturon treatment or combination of isoproturon and dicamba or mixing between 2,4-D, isoproturon and surfactant provided the maximum control of C. album in wheat (Malik et al., 1992). Efficacy of herbicide treatment with thifensulfuron for C. album in soybean field was great (Monks et al., 1993). Mixing between bentazone and imazethapyr can control both of redroot pigweed (Amaranthus retroflexus) and C. album in common bean (Phaseolus vulgaris) (CABI, 2018).
Biological control
Although chemical herbicides are quite effective to providing immediate control, however it can cause numerous harms to the environment, humans and off target organism. The biggest concern when using chemical herbicides is their residues that may persist for long periods in soil and aquatic environments and can cause damage to plants and animals. Humans can also be affected by exposure through eating herbicide-treated food that is extremely harmful before completion of the waiting period, leading to serious illness (Le et al., 2015). Moreover, repeated use, increase of the effective dosage, and the excessive use of herbicides may lead to create herbicide-resistant biotypes (Bo et al., 2019). International survey of herbicide- resistant weeds (2018) reported that C. album become herbicide-resistant weed in 20 countries in the world including Belgium, Bulgaria, Canada, Czech Republic, Finland, France, Germany, Greece, Italy, Netherlands, New Zealand, Norway, Poland, Portugal, Slovenia, Spain, Sweden, Switzerland, United Kingdom, United States and it has not been found in Korea yet.
Therefore, there is a need to develop biological control for their effective control and contribute largely to sustainable agriculture. Biological weed control is an approach using living organisms to control or reduce the population of a selected, undesirable, weed species, whilst leaving the crop unharmed (TeBeest et al., 1992). When successful, biocontrol can be an environmentally-friendly powerful tool over large areas (Gurr and Wratten, 2000; Kim et al., 2018). Generally, most bio-herbicides have no effect on non-target organisms, are not poisonous to mammals and do not contaminate soil or groundwater (TeBeest and Templeton, 1985). Research in Europe has explored Ascochyta caulina, a proposed microbial herbicide (mycoherbicide) for biocontrol of the weed C. album (Kempenaar, 1995). Ascochyta caulina is a plant pathogenic fungus, which is specific to C. album, causing necrotic lesions on the leaves and stems. A. caulina is a well-known toxin-producing genus. Members of this genus produce a range of phytotoxin compounds that are chemically diverse and possess a broad range of biological activities. Phytotoxins might well be responsible for the necrosis development of C. album plants by A. caulina (Scheepens et al., 1997). C. album has been identified among targets for future research into the potential for biological control (Schroeder et al., 1993). Researchers found that up to 70% control was achieved in field conditions (Scheepens et al., 1997).
Allelopathy
Researchers have focused on seeking suitable safe natural compounds as an alternative source which are essential for weed management. Under ecological approaches, use of the antagonistic plants, natural plant-based formulations and extracts of natural plant products in purified and even the volatile essential oils have been proposed as a feasible option for alternative weed management in sustainable agricultural systems. Depend on the specificity or selectivity of the allelopathic plant to some weed species (target species). Several plants release allelopathic exudates from living tissues or by decomposition of plant residues which influence other plants in their surrounding vicinity (Duke et al., 2002; Einhellig, 1996; El-Shahawy, 2010; Inderjit, 1996; Putnam, 1988).
Synowiec and Drozdek, (2016) reported peppermint (Mentha × piperita L.) oil and caraway (Carum carvi L.) oil were generally herbicidal against C. album. The phytotoxic effects of these oils could be attributed to the presence of the constituent carvone and limonene (caraway oil) and menthone and menthol (peppermint oil), and to the chemical groups monoterpenes and terpenes (Synowiec and Drozdek, 2016). Moreover, the essential oils obtained from rosemary (Rosmarinus officinalis L.), thyme (Thymus vulgaris L.), and savory (Satureja montana L.) have allelopathic potential with C. album (Luciana et al., 2003).
Dogan et al. (2016) indicated that allelopathic potential from extract of mint (Mentha piperita L.), thyme (Thymus vulgaris L.), rosemary (Rosmarinus officinalis L.), coriander (Coriandrum sativum L.) and sage (Salvia officinalis L.) could be used as alternatives herbicides, which can inhibit seed germination, seedling and root growth of C. album.
Conclusion
C. album is an exotic weed with serious environmental and economic consequences. Although, nowadays there is appreciable progress in the area of C. album management through various methods, but its ecology still needs to be explored. Therefore, scientists need to assess the impact of exotic weed at several levels of ecological enhancement in order to gain influence in their management and control. In sustainable agricultural training, agricultural extension staffs should discuss with farmer about ecological hazards of this weed, thereby it will contribute significantly to weed management. In addition, arrangement and focus on extracurricular classes for students are important to know about basic knowledge on biology and ecology of plants including weeds, thereby students can recognize exotic weeds from a seedling stage and remove them at an early period.
Acknowledgements
This work was carried out with the support of the “Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ01321601)” Rural Development Administration, Republic of Korea.
Authors Information
Kee Woong Park, Chungnam National University, Professor
Botir Khaitov, Chungnam National University, Researcher
Kwang Min Cho, Chungnam National University, Researcher
Thi Hien Le, Chungnam National University, Ph.D. student
Weiqiang Jia, Chungnam National University, Ph.D. student