Introduction
Transgenic canola (Brassica napus) known as a major source of oil, and is a crop in which the risk of unintentional release of unwanted gene or genes from volunteers to subsequent non-GM or feral populations and its occurrence and frequency had been broadly analyzed. Occurrence and frequency of transgenic volunteers strongly link with the amount of seed added to soil. Hence, perception the seedbank ecology of canola and factors that contribute secondary dormancy are of significance to reduce the concerns related to the seed bank persistence of this species. Persisting seed bank of canola can be accumulated in the soil due to seed loss before and during the harvest in cultivating (Canada, Australia, etc.) countries (Baker and Preston, 2003; Simard et al., 2002) and transportation in importing (South Korea, etc.) countries (Lee et al., 2010; Park et al., 2010). Other mechanisms such as ungerminated seed from sowing, seed produced from volunteers or seed loss from mature plants as a result of heavy rainfall, hail, strong winds or lodging responsible to build up the seed bank of canola.
Canola seed has almost no dormancy at the maturity stage (Lutman, 1993; Pekrun et al., 1998). However, non-dormant seed could convert dormancy unless environmental conditions are compatible for germination. This process is called “Secondary dormancy”. Secondary dormancy plays a role of persistence mechanism in the seed and takes place as a response to unfavorable conditions (Gulden et al., 2000; Linder, 1998; Pekrun et al., 1997b; Pekrun et al., 1998). Secondary dormancy in canola might extend persistence of seed bank but there is a variation among genotypes (Pekrun et al., 1997c). Secondary dormancy can be broken by introducing the low temperature (2-4℃) (Gulden et al., 2004) or by alternating warm and cold temperatures (Pekrun et al., 1998).
Persistence of canola seed varies in disturbed and undisturbed soils. Deterioration is considerably faster in cultivated soils compared to non-cultivated ones (Chadoeuf et al., 1998). Masden (1962) revealed that seeds of canola could keep viable for at least 5 years in disturbed soils and that up to 16 years at around 20 cm depth of undisturbed soil. Crawley et al. (1993) experimented to determine the viability of herbicide-tolerant GM canola and non-GM canola seeds after time of burial in the UK. Transgenic canola showed no remaining viable seed after two years of burial at 2 and 15 cm depth of soil, while 0.5% of seed from initial non-GM seed kept viable. Another study in France determined that at 30 cm depth of soil, viability of canola seed ranged from 0.03 to 0.08% after 36 months of burial and that at 41 months, the canola seed was non-viable (Chadoeuf et al., 1998).
Canadian study determined that density of canola seedbank reduced ten times in the first year but there was a gradual decrease subsequently (Legere et al., 2001). Seasonal variations in seedbank density may be taken place by volunteer plants, which produce mature seeds every year thereby refilling the stock of canola seeds in the soil. Another field monitoring carried out in Canada made known, although at low density, volunteer canola plants have been found in fields four to five years after canola crop cultivation (Legere et al., 2001; Simard et al., 2002). Harker et al. (2006) investigated the persistence of canola seed under controlled conditions where no way to replenish the seedbank, viable canola seed was not observed after three years of experiment.
The persistence of canola seed in the seed bank significantly depends on vertical distribution of seed in the soil. Infield experiments, the effect of burial depth on the persistence of seed bank had a fixed pattern but generally, seed viability was inferior. Seeds are more likely to persist at deeper soil depths than at shallow depth in the field (Pekrun et al., 1998). A greater proportion of germination occurs at shallow depths burial due to dormancy-breaking condition alterations such as lying open to light and temperature (Pekrun et al., 1997a, Pekrun et al., 1998). Some proportions of the seed (around 1.5%) which were buried at 5 cm depth of soil emerged during 5-6 weeks. Nearly 80% of the initial seed taken from the soil was dead seed and just 0.6% of seed could keep viable after 7 months of burial. Similar result was obtained when the seed placed to a depth of 20 cm but somewhat it was variable. Some of seed (0.07 to 0.5%) buried to a depth of 20 cm germinated after up to one year of burial (Lutman, 1993). Pekrun et al. (1997c) revealed that the major contributing factors to the persistence of the seed bank of canola are dark conditions and physical and chemical properties of soil. Clay soil, with high water keeping capacity tended to show lower proportion of dormant seeds in the canola seed bank than sandy soil, which has low water holding capacity (Pekrun et al., 1998).
Canola seedling emergence might be influenced significantly by burial depth. The available soil moisture, oxygen and light affect the fate of seed in the soil (Benvenuti, 2007). Under similar value of moisture, oxygen and light, number of emerged seedlings could decline as soil depth increases (Colbach et al., 2006; Gardarin et al., 2010; Sester et al., 2007). No change was observed on seedling emergence from 1 cm to 2.9 cm depth of burial shoving a value of 98.4% (Soltani et al., 2013). Emergence was never observed beyond 10 cm (Soltani et al., 2013) or 12 cm (Colbach et al., 2008) depth of soil. Soltani et al. (2013) reported that a failure to emerge could be resulted by pre-emergent mortality or insufficient germination. Buried seeds in deeper soil layers require more seed reserves than shallow depth to get emergence. There are a number of studies conducted on the emergence of canola as burial factor is concerned. However, it should be examined the effect of burial depth on the emergence of Brassica napus not only under greenhouse conditions, but under certain field conditions as well.
We conducted experiments to investigate the effects of duration and depth of burial on seed viability, seasonal germination, primary and secondary dormancy, and seedling emergence of canola seeds.
Materials and methods
Seed persistence and viability
Experimental site and seed burial preparation
The experiment was conducted at the confined field experimental station of Chungnam National University, Daejeon, South Korea. Canola seeds were buried in the first decade of April (beginning of the season) and July (maturity) in 2018. One hundred seeds were counted and enclosed in a 5 cm square, 60-micron nylon mesh pocket, with a plastic bead color coded for each canola line and it was secured to a cord. The openings in the mesh were large enough to allow the movement of air, water and microorganisms. The thread was attached firmly perpendicular to a stake, with packets suspended at 5, 25, and 30 cm from the underside of the stake. Eight removal dates were replicated three times. Control samples were maintained at 13±5℃ under dark condition in the laboratory. Holes were spaced 50 cm apart and dug to a depth of approximately 40 cm. Stakes were placed perpendicular to the hole, allowing the cord to hang freely. The holes were then filled until level with the underside of the stake.
Seed removal and germination
Seeds were exhumed 2, 3, 4, 5, 7, 12, 15, 18 months and 1, 2, 4, 8, 12, 15 months after burial in April and July respectively. The hole was dug 10 cm apart from the original hole to a depth of 50 cm. Soil was removed with hand from around the cord. The thread with packets attached was removed from the stake, and washed the remaining soil and debris gently with water. Following by, the threads and pockets were air-dried for about one hour prior to counting and planting. Thereafter, the number of intact seeds was counted and then the data was recorded. To determine the germination, intact seeds were planted into water-soaked filter paper in a 60×15 mm Petri dish (SPL Life Sciences Co., Ltd., Pocheon, Korea). For comparison the germination rate between buried and unburied seeds, the stored sample, which were kept in the lab (around 13℃ on the shelf) under non-influence of environmental and burial factors, was used. Seeds were germinated in an incubator (Hanbaek Co., Ltd., Pocheon, Korea) at 25℃ (13/11 h) light and dark photoperiod for 10 days. Germination was defined as the emergence of a coleoptile from the seed.
Seed viability test
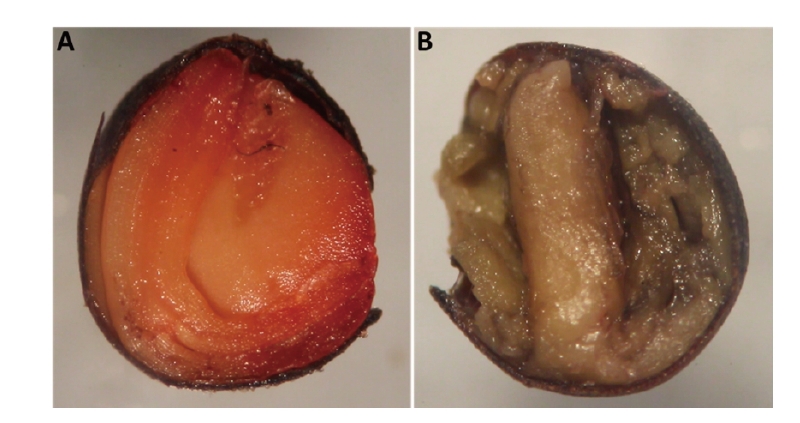
To determine the viability of non-germinated intact seeds, tetrazolium test was carried out and following step determinations were performed:
Pre-conditioning–soaking in a humidified paper towel for 8 hours at 25℃.
Preparation of the seeds-longitudinal incision through the integument and cotyledons.
Coloration–longitudinal incision seeds were placed in a 60×15 mm Petri dish (SPL Life Sciences Co., Ltd., Pocheon, Korea), in a dark environment for 6 hours in a 0.5% triphenyltetrazolium solution at 25℃.
The Stemi DV4 Stereo Microscope (Carl Zeiss, Göttingen, Germany) was used to evaluate the viability of each seed. Number of viable seed was recorded and used for ultimate data analyses.
Identification of emergence rate by soil depth
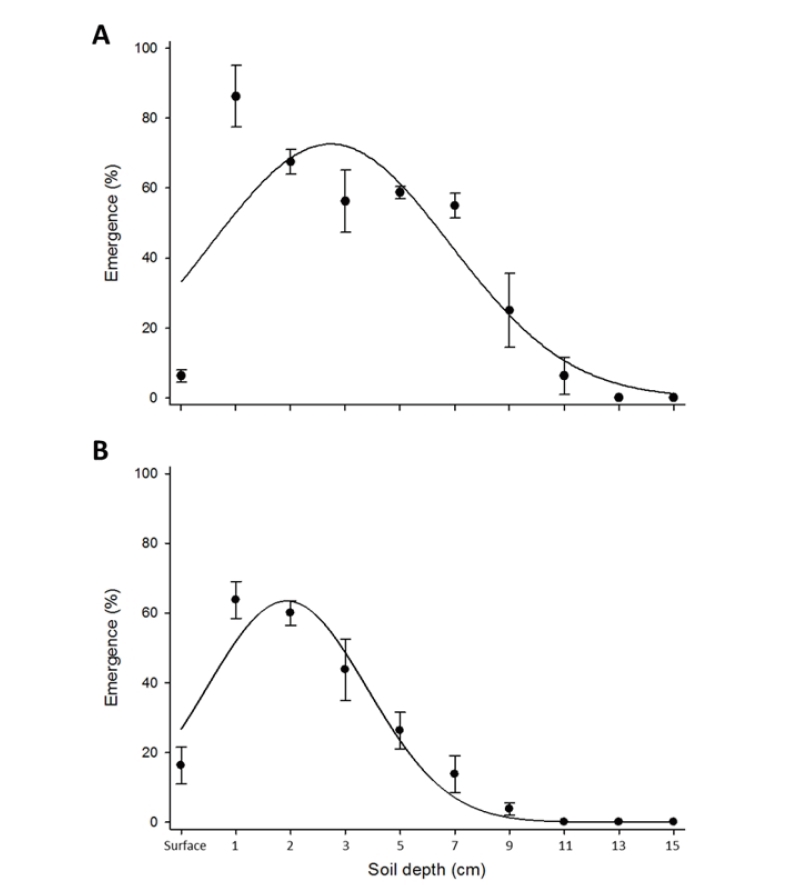
Emergence rate of canola according to different depth of soil was identified under glasshouse and field conditions in March and June 2019 respectively. Firstly, the mixture (1:1 (w/w) of horticultural nursery soil (Bunong, Korea) and rice nursery soil (Bunong, Korea) were placed into the plastic pots (20.0 cm inside diameter, 19.0 cm height, 0.5 cm thickness). Then, 40 canola seeds were sown at the surface, 1, 2, 3, 5, 7, 9, 11, 13, and 15 cm depth of soil with three replication. Number of seedlings was counted and recorded 21 days after seeding (DAS). Regarding the field experiment, 50 cm2 plots were prepared with thee replication and 40 seeds of canola were seeded at the surface, 1, 2, 3, 5, 7, 9, 11, 13, and 15 cm depth of soil. 28 DAS number of emerged seeds was counted and recorded.
Data analyses
An exponential decay curve was fitted to the intact seed fraction to estimate the deterioration of seeds. Estimated seed half-life of buried canola lines at all burial depths was determined using the following equation:
Wherein, y is the proportion of seeds remaining in the soil; a is the initial number of seeds buried, b is the decay factor, and x is the amount of time that has passed in months.
Results and discussions
Seed persistence and viability
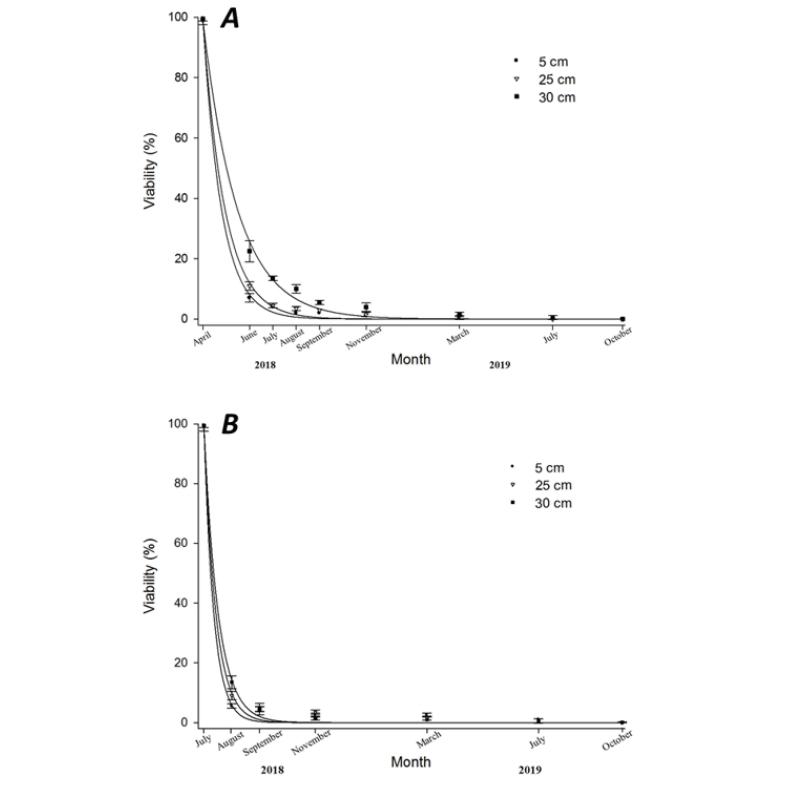
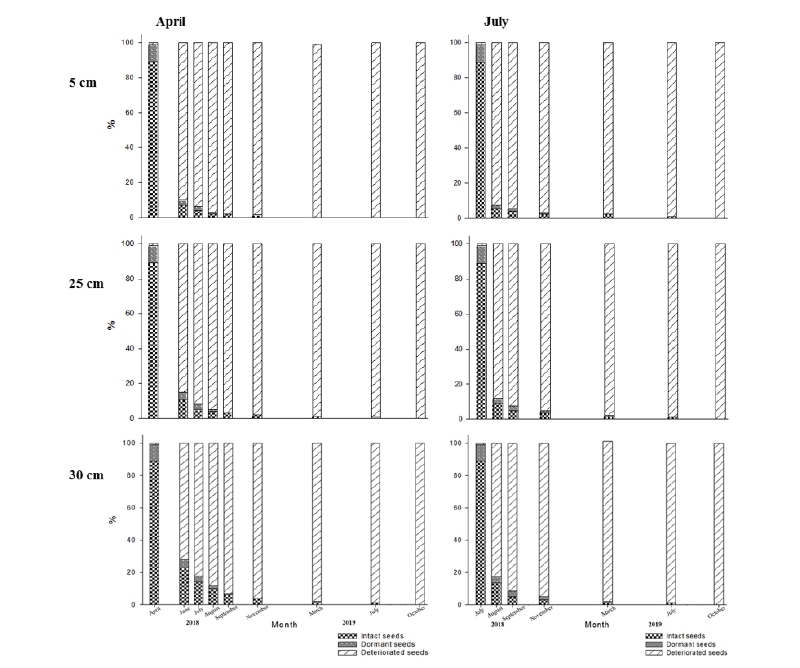
In our experiment, the effect of burial depth on seed viability varied but generally, as time passed, the seed viability was extremely low. The seeds, which were buried in July 2018 deteriorated quicker than those buried in April 2018 (Fig. 1, 2, and 3). Low soil temperature, lack of microorganisms and environmental factors such as heavy rainfall and/or sunlight intensity are the main influencer factors that might make deterioration slow. As Pekrun et al. (1998) report, the seeds are more likely to persist at deeper soil depths than at shallow depth in the field. This hypothesis has been proved in our experiments. The seeds, which were buried at 30 cm depth could persist longer than the seeds had been buried at 5 and 25 cm. It could be explained by the fact that factors especially soil temperature, number and types of microorganisms, sunlight intensity and so on are lesser at 30 cm depth. As seeds were buried longer period, intact and dormant seeds were less and deteriorated seeds were more. 6.8% (April) and 4% (July) of initial seeds buried to a depth of 5 cm could germinate after two months of burial and that only 0.5% and 0.4% of seed recovered as whole seed after12 months of burial. Seeds placed at 25 cm and 30 cm depth showed quite similar results at the end of the burial period. Most of the seed buried to a depth of 25 cm had a survival rate ranging from 0.8% to 1.0% after up to 12 months of burial in both burial timing. Canola seed could not persist more than 15 months if they were buried at 5 cm and 25 cm depth of soil. While viable seeds were still observed when the seeds had been left 30 cm depth of soil. Regarding the dormancy, canola seeds more likely tended to show a higher level of dormancy at the deeper layer of soil profile. Dormancy level was 10% at the beginning of the experiment (before burial). However, four months after burial, no dormant canola seeds were observed at all burial depth and in both burial timing (Fig. 3).
Identification of emergence rate by soil depth
Quantification of seed emergence as influenced by burial depth was performed satisfactorily, and it was revealed that the greatest number of canola was observed when seeds were at 1 cm depth of soil under both greenhouse and field conditions (Fig. 5). Canola seeds are not too big (1,000 seeds weight raging around 4-6 g). 1 cm burial depth is optimum depth for canola to emerge properly on time and it makes germination level and growth parameters higher. Under field conditions, rainfall and other environmental factors played as a role of enhancer that compared to the greenhouse condition. Noticeably, more canola seedlings were recorded when the seeds were sown at the soil surface under open field condition (Fig. 5B). There are several reports on canola emergence as affected by seeding depth: Gruber et al. (2010) reported that the emergence of canola was the highest at soil depths of 1-5 cm, whereas it was dropped significantly at depths of 0 and 7 cm, and emergence was completely inhibited at 12 cm. In our study, a dramatic decrease was observed at the soil depth from 2 cm to 10 cm and canola seeds did not emerge beyond 13 and 11 cm depth of soil under greenhouse and field conditions respectively (Fig. 5).
Overall, the pattern of seedling emergence and persistence of buried seeds have been introduced as two important factors determining the success of canola seed (Figueroa et al., 2007; Momoh et al., 2002). In this study, it was observed that the level of seedling emergence and the persistence of canola seedbank were affected by burial depth and burial timing.
Conclusion
Based on the seed burial study, it would be concluded that the seeds, which were buried at 30 cm depth of soil, could persist longer than the seeds had been buried at 5 cm and 25 cm. As seeds were left longer period, intact and dormant seeds were less and deteriorated seeds were more. Seeds, which were buried in July 2018, deteriorated quicker than those buried in April 2018. Low soil temperature, lack of microorganisms and environmental factors such as heavy rainfall and/or sunlight intensity are the main influencer factors that might make deterioration slow. Emergence rate of canola was differed depending on the depth of burial. The greatest number of canola was observed when seeds at 1 cm depth of soil under both greenhouse and field conditions. Canola seeds did not emerge beyond 13 and 11 cm depth of soil under greenhouse and field conditions respectively. The results of this study will also be useful for future studies of biology and ecology of transgenic canola. However, the persistence of viable canola seed in the soil under Korean field conditions is barely understood and further research is needed.
Acknowledgements
This work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through Agri-Bio industry Technology Development Program funded by the Ministry of Agriculture, Food and Rural Affairs (MAFRA) (317073-02-2-HD030).
Authors Information
Kee Woong Park, Department of Crop Science, Chungnam National University, Professor, https://orcid.org/0000-0003-0053-9543
Cho Kwang Min, Department of Crop Science, Chungnam National University, Researcher, https://orcid.org/0000-0003-0537-2164
Botir Khaitov, Department of Crop Science, Chungnam National University, Researcher, https://orcid.org/0000-0001-6314-1597
WeiQiang Jia, Department of Crop Science, Chungnam National University, Ph.D. student, https://orcid.org/0000-0002-1654-3638
Mirjalol Umurzokov, Department of Crop Science, Chungnam National University, Master student, https://orcid.org/0000-0001-8148-8041
Jin-Woong Cho, Department of Crop Science, Chungnam National University, Professor, https://orcid.org/0000-0002-4263-8278
Soo-In Sohn, National Institute of Agricultural Sciences, Biosafety Division, Researcher