Introduction
Algae is a diverse group of prokaryotic and eukaryotic organisms capable of producing oxygen through photosynthesis. Because of its biological characteristics to produce extra-cellular materials that bind soil particles together, it provides organic matter to the soil and reduces soil erosion (Bond and Harris, 1964). Despite these benefits to agricultural soil, problematic algae has been found in areas where turf is associated with unfavorable conditions for growing healthy and dense turf resulted from pest infestations or physiological stress. Among various types of algae, blue-green algae, a prokaryote and often called cyanobacteria which is theoretically in separate taxonomic kingdoms, is mostly found in turf (Elliott, 1997; Raven et al., 1981; Turgeon and Vargas, 2006). Blue-green algae is known to cause two distinct problems on turfgrass which are surface slime mats and subsurface black layer (Baldwin and Whitton, 1992). The slime mats are described as the layers that interrupt the playing surface and inappropriate soil condition for turf growth. When the slime mats dry, it creates a crust that is impervious to water that can induce subsurface black layer (Turgeon and Vargas, 2006). Several research results regarding negative effects of blue-green algae on turfgrass growth have been reported with surface black layer, toxicity of black-layer inducted by cyanobacteria, algaecide tolerance of blue-green algae, and blue-green algae associated with yellow spot disease (Baldwin and Whitton, 1992; Elliott, 1998; Hodges and Campbell, 1997; King and Ward, 1977; Maddox et al., 2001; Tredway et al., 2006). Because algae has been problematic to turf industry, many chemical algaecides have been evaluated for appropriate turf management.
Inguagiato et al. (2018) used phosphite to control cyanobacteria on creeping bentgrass (Agrostis stolonifera) which is the substitution of an oxygen atom for a hydrogen. The research results reported that phosphites applied at rates of 0.54 g m-2 suppressed cyanobacteria with minimal risk of phytotoxicity on creeping bentgrass greens. But greater rate of phosphites than 1.37 g m-2 produced phytotoxicity on turfgrass leaves. Elliott (1998) evaluated four chemicals to control cyanobacteria on bermudagrass (Cynodon dactylon) putting green which are mancozeb, maneb, chlorothalonil and quaternary ammonium salts. The research concluded that all treatment except quaternary ammonium salts suppressed blue-green algae effectively as preventive applications but they were not effective to control blue-green algae as curative application. Chlorothalonil labeled for turfgrass has been reported algae control from the previous researches (Gazstonyi and Lyr, 1987; Smith and Goulding, 1972). Copper and zinc are essential micronutrients for algae enzyme systems. However, excessive concentrations of copper and zinc can suppress algae growth (Cavet et al., 2003; Franklin et al., 2002; Stauber and Florence, 1990; Stauber and Florence, 1987). To control cyanobacteria, the proper rate of the copper or zinc application is required because the chemicals including copper or zinc can be released from chemical compounds to provide nutrients leading to next algae occurrence.
Hydrogen peroxide (H2O2) has been used to control algae on surface water. Hydrogen peroxide is known as more effective algaecide to control blue-green algae than green algae on surface water. Barroin and Feuillade (1986) reported that 1.75 ppm of H2O2 is needed to significantly reduce the cyanobacterium, Planktothrix rubescens compare to the green algae Pandorina morum which need 10 times greater amount of H2O2. Matthijs et al. (2012) investigated H2O2 as an algaecide to control blue-green algae on surface water. Based on their results, they reported that the use of dilute H2O2 to control harmful cyanobacteria from recreational lakes and drinking water reservoirs is recommended when immediate control is required. Although the benefits to control blue-green algae, the H2O2 application to natural waters is often considered as one of the factors to induce environmental pollution on water sources. However, H2O2 does not exist for long term under natural condition because H2O2 is rapidly decomposed into water and oxygen. H2O2 is also produced in nature of all surface water and most organisms produced H2O2 for their biological metabolism. (Asada, 2006; Cooper and Zika, 1983; Veal et al., 2007). Therefore, the preconception about H2O2 applications to nature is not necessary if the rate of H2O2 applications does not exceed the optimal rate. Sodium bicarbonate (NaHCO3) is commonly used for biomass and carbohydrate production of cyanobacteria as new biofuels because it is a source of inorganic carbon for photosynthetic microorganisms (White et al., 2012). Paradoxically, sodium bicarbonate for turfgrass management has been reported as a material to control moss on creeping bentgrass putting green (Burnell et al., 2004; Kennelly et al., 2010). However, there are gaps in research results regarding algae control treated by sodium bicarbonate. Sodium hypochlorite (NaOCl) is a chemical compound known as liquid bleach, which is widely used as a household chemical and an oxidizing agent for organic products. Colbaugh and Metz (1994) evaluated five different combinations of algaecide materials on bermudagrass putting green. They reported that sodium hypochlorite is effective to control algae when it is used with Latron CS-7 (blend of alkyl polyethoxylate and sodium salt of alkylsulfonated alkylate+silicone antifoam compound, 60% active ingredient) at 30 mL L-1. The optimum rate of sodium hypochlorite and Latron CS-7 is required to minimize phytotoxicity on turf leaf. Phytotoxicity to bermudagrass treated by higher rates of sodium hypochlorite when mixed with surfactant has been reported (Colbaugh and Williams, 1993; Colbaugh et al., 1994).
To establish turfgrass lawn, there are many factors such as seed selection, temperature, soil types, nutrient levels, irrigation program, and methods to establish (Christians, 2011). While turfgrass seeded is germinated and established, infestation of algae may be a negative factor, decrease the rate of germination, and delay the time of establishment. Although the many previous researches have been reported about algae control by chemical materials as stated, there are little research results available regarding the algae controls by chemical applications during the time of turfgrass establishment. The research objective is to compare chemical materials to control algae during establishment of Kentucky bluegrass.
Materials and Methods
Research was initiated at the Hoseo University in Asan, Chungnam, South Korea. The 20 g m-2 of seeding rate was applied for Kentucky bluegrass ‘Midnight’ establishment on a 12-cm diameter pot and managed to reach 50% coverage which is taken 23 days. All pots with Kentucky bluegrass seeded were managed in growth chamber (GC-1000, Jeio Tech. Daejeon, Korea) for consistent light condition, humidity (80%) and air temperature (day/night, 25/20℃) (Zhang et al., 2013). Photosynthetic light condition (400-700 nm) and photoperiod were 300 mol m-2 s-1 and 16 h (Qian and Chieri, 2009). The complete fertilizer (Super 21, 21-17-17, Namhae Chmistry. Inc., Yeosu, Korea) and phosphate fertilizer (Yongsunginbi, 0-17-0, Poongnong Inc., Seoul, Korea) was applied at the level of 5 g m-2 for each product before the initiation of the study.
Algae was harvested from the soil surface of Kentucky bluegrass lawn located in Hose University Turfgrass Research Station. Harvested algae were collected by scraping off the soil surface and then added to water container with the complete fertilizer at the rate of 10 g m-2. Harvested and collected algae was tested to identify using jar test and identified as blue-green algae (Austin et al., 2018). The water container with algae is stored in the growth chamber at a temperature of 28℃ and 90% humidity to increase the population of algae (Bernstein et al., 2014). Grown algae is added to each treatment plot at the rate of 25 mL per pot after stirring algae solution for the uniformity. The 25 mL of algae solution covered 50% of the 12-cm diameter pot. The sodium hypochlorite (4% NaOCl, Yuhan Clorox Regular®, YUHANCLOROX Ltd., Seoul, Korea) was used at the rate of 250 and 500 mL L-1 as low and high rates, respectively. The hydrogen peroxide (35% H2O2, Green Hydrogen Peroxide®, JK Medi. Inc., Yeoju, Korea) was used at the rate of 3 and 6 mL L-1 as low and high rates, respectively. The sodium bicarbonate (100% NaHCO3, Heige Plus Baking Soda®, Advanced Chemical Inc., Yongin, Korea) was used at the rate of 40 and 80 g L-1 as low and high rates respectively. The iron sulfate (20% FeSO4, NoGreen®, De-AN Tech. Inc., Geumsan, Korea) was used at the rate of 10 and 20 mL L-1 as low and high rates, respectively. The first treatment was applied at February 9, 2019 when turf coverage reached 50%. The treatment list is in Table 1. After the treatment, turfgrass quality, turfgrass coverage, algae coverage, and clipping dry weight were measured weekly. Turfgrass quality was measured by an index of turf density, leaf texture, disease resistance, mowing qualities, and stress tolerance including several stresses during establishment (Beard, 1973; Turgeon, 2008). Turfgrass quality was visually rated on a scale of 1 to 9 (1=poor, 6=acceptable, and 9=best). The percent area of turfgrass and algae coverage was measured by visual evaluation (NTEP, 2011). Clippings were collected every week from each plot at the 3 cm of mowing height, dried at 67℃ for 48 hours using a dryer (HDG-222HR, Hyundai EnerTec. Co., Ltd., Hwaseong, Korea) and weighed to be calculated for clipping dry weight.
|
Table 1. Treatment list of chemical materials and rates. 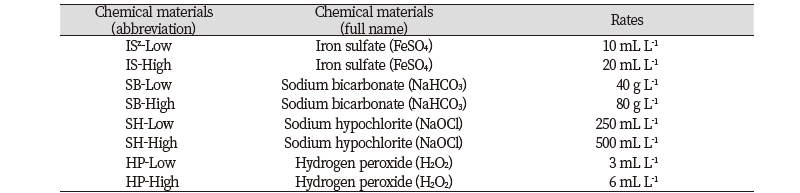
|
|
zIS: Iron sulfate; SB: Sodium bicarbonate; SH: Sodium hypochlorite; HP: Hydrogen peroxide. |
The study was set up as a randomized complete-block design and treatment structure was a two (application rates) × four (chemical materials) factorial plus a control that received no chemical applications with three replications. The data were subjected to a one-way analysis of variance (ANOVA) in PROC ANOVA (SAS Institute, 2003). For interaction observation and main effect analysis, data were subjected to a two-way ANOVA in SAS GLM and separated at a 0.05 probability level according to the Fischer’s protected least significant difference (LSD) at a 0.05 probability level.
Results and Discussion
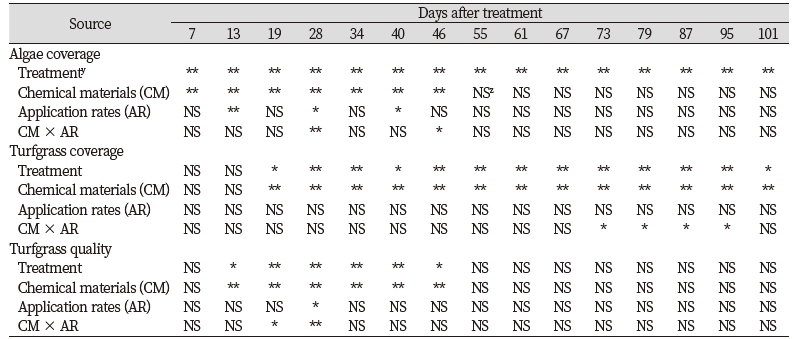
There were no consistent two-way interactions between chemical materials and application rates for algae coverage, turfgrass coverage, and turfgrass quality (Table 2). All chemical applications had significant effects on algae coverage compared to control from 28 to 101 days after treatment (DAT) (Table 3). The control plot had the greatest algae coverage from 28 to 101 DAT. The sodium bicarbonate (SB) had no differences with control until 19 DAT. Significant differences between iron sulfate (IS) and control were found during all rating dates. The sodium hypochlorite (SH) had significant effects compare to control for all rating dates with the exception of 7 DAT. Between the hydrogen peroxide (HP) and control, significant differences were found on algae coverage after 13 DAT. Between low and high rates, significant differences were found only for the SB from 34 to 55 DAT and the HP at 28 DAT. Overall, no difference were found between application rates based on one-way analysis of variance.
Significant differences for application rate main effects on algae coverage were found only on three of 15 rating dates (Table 2). There were significant chemical materials main effects for algae coverage from 7 to 46 DAT. The IS had the greatest or equal to the greatest reduction of algae coverage when significant differences were found for chemical material main effects (Fig. 1). The IS had the lowest algae coverage and decreased algae coverage about 74% on 7 DAT. According to Holmes (2002), the effects of chemical applications to control algae appeared immediately compare to natural materials such as barley straw which takes one to three months to control algae. As reported from the previous research, chemical materials are had quicker effects than natural materials. Among chemical materials evaluated under the condition of this study, the IS had the fastest control of algae during turfgrass establishment. When the immediate control of algae during the establishment of Kentucky bluegrass is required, the IS can control algae with 74, 87.8, and 92.1% reduction of algae coverage within 7, 13, and 19 days, respectively based on the results of the study. In contrast to the IS, the SH, and SB treatment had 12.4 and 2.1% reduction of algae coverage at 7 DAT. After 19 DAT, the IS had no differences with the SH. The SH produced the second greatest reduction of algae coverage of the reduction of 54.1 and 73.4% on 13 and 19 DAT, respectively. The SB had the lowest or equal to the lowest reduction of algae coverage when significant differences were found. Ranking of chemical materials to reach greater than 70% reduction of algae coverage in time was IS (7 days) > SH (19 days) > HP (34 days) > SB (46 days). However, there were no significant differences for chemical main effects on algae coverage after 46 DAT.
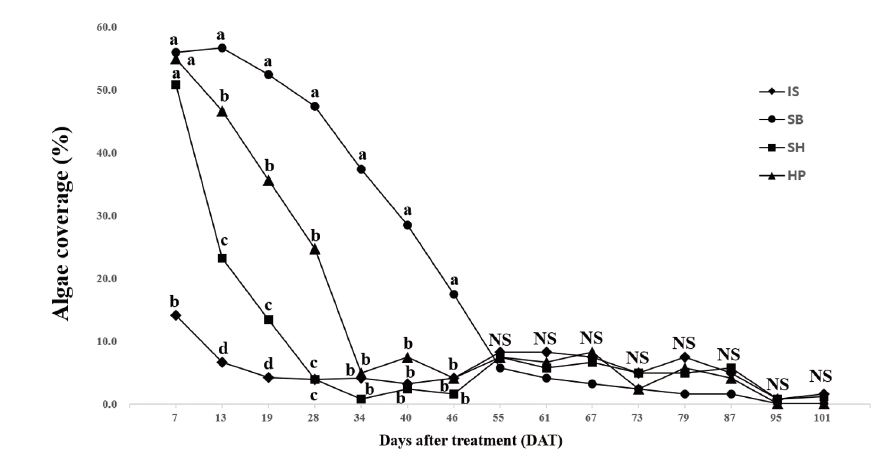
Fig. 1. Mean algae coverage (%) per unit surface of ‘Midnight’ Poa pratensis by the chemical material main effect. Each mean was calculated from six observations at four chemical materials (main effect averaged over two application rates with three replications). Means with the same letter within each DAT (days after treatment) or denoted (NS) are not significantly different according to Fisher’s LSD (P=0.05). IS: Iron sulfate (FeSO4); SB: Sodium bicarbonate (NaHCO3); SH: Sodium hypochlorite (NaOCl); HP: Hydrogen peroxide (H2O2).
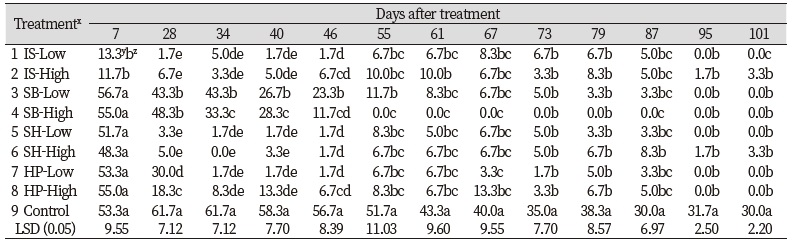
No differences were found for turfgrass coverage until 13 DAT among all chemical treatments and control (Table 2). The SH had lower turfgrass coverage than control when significant differences were found in one-way analysis of variance (Table 4). This reduction of turfgrass coverage may be resulted from the excessive application rate of chemical materials that can possibly induce phytotoxicity. Sodium hypochlorite has been reported for effective algaecide by previous researches when it is used at the rate of optimum concentration. Colbaugh and Williams (1993) reported that sodium hypochlorite combined with surfactant had the equal to the greatest effects on algae control on bermudagrass (Cynodon dactylon) golf course green. However, sodium hypochlorite combined with surfactant leaded to the greatest phytotoxicity among all treatments. According to Vincelli et al. (2017), the high rate of chemical fungicide can induce undesirable symptoms including a coarser appearance through a widening of leaf blades, reduced growth rate, and color changes. Both low and high rates of SH used in the study may be greater than optimum concentration that can induce phytotoxicity during establishment of Kentucky bluegrass. There were no significant chemical materials × application rates interactions for turfgrass coverage with the exception of the rating dates from 73 to 95 DAT (Table 2). No significant differences for application rates were found on turfgrass coverage during the period of the study. There were significant differences for chemical materials main effects on turfgrass coverage on 13 of 15 rating dates. For the main effects of chemical materials, the SH had the lowest turfgrass coverage when significant differences were found (Fig. 2). As stated, the SH produced greater than 70% reduction of algae coverage within 19 days. However less than 50% of Kentucky bluegrass coverage was produced with the SH treatment during the period of the study. The SH reduced 76.7% algae coverage on 19 DAT but turfgrass coverage was 26.7% at the same rating date (Table 3 and Table 4). In comparison with the SH, the IS and SB had the greatest or equal to the greatest turfgrass coverage from 19 to 101 DAT. Kentucky bluegrass treated by the IS had the greatest turfgrass coverage and reduction of algae coverage during the study.
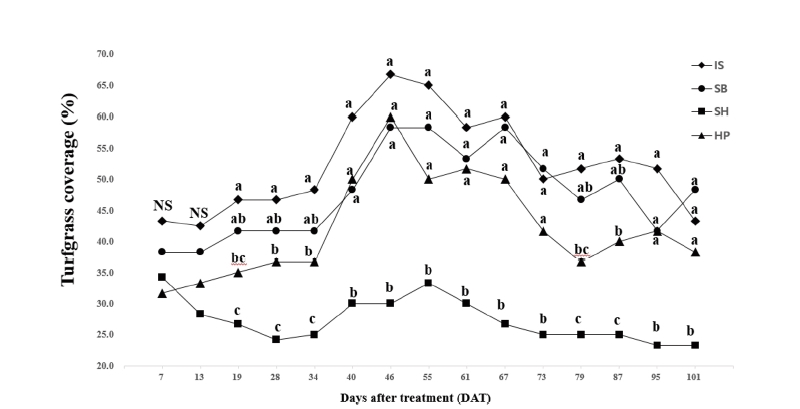
Fig. 2. Mean turfgrass coverage (%) per unit surface of ‘Midnight’ Poa pratensis by the chemical material main effect. Each mean was calculated from six observations at four chemical materials (main effect averaged over two application rates with three replications). Means with the same letter within each DAT (days after treatment) or denoted (NS) are not significantly different according to Fisher’s LSD (P =0.05). IS: Iron sulfate (FeSO4); SB: Sodium bicarbonate (NaHCO3); SH: Sodium hypochlorite (NaOCl); HP: Hydrogen peroxide (H2O2).
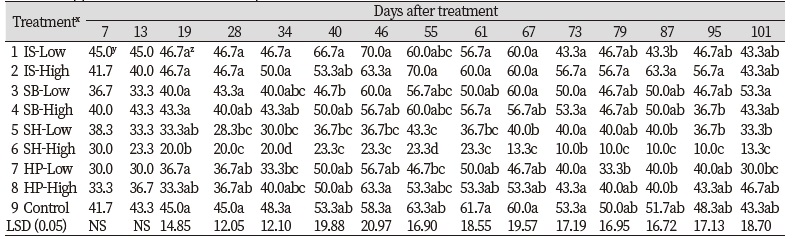
All treatment of chemical materials had no differences with control except the SH. Only the SH had turfgrass quality equal to six or less than six which is acceptable quality when significant differences were found based on one-way analysis of variance (data not indicated). There were significant chemical materials × application rates interactions for turfgrass quality on 2 of 15 rating dates. No significant differences for application rate main effects were found on turfgrass quality for all rating dates. There were significant chemical material main effects for turfgrass quality on 6 of 15 rating dates. Among the treatments of chemical materials except the treatment of SH, no significant differences were found for turfgrass quality on all rating dates. Three treatments of IS, SB, and HP had quality ratings greater than the acceptable rating of six throughout the research (Fig. 3). The SH had quality ratings lower than the acceptable rating of 6 when significant differences for chemical material main effects were found on turfgrass quality. Sodium hypochlorite have often used to increase germination rates of plant seed (Kim et al., 2005). Haynes et al. (1997) evaluated switchgrass (Panicum virgatum) treated by various chemical materials for germination and seedling emergence rates. They reported that switchgrass seeds treated by sodium hypochlorite had 1.8 to 1.9 times greater emergence rate than non-treated. Similar to turfgrass coverage with the treatment of sodium hypochlorite, low turfgrass quality may be resulted from the excessive rates of application and duration of exposure time to sodium hypochlorite. In previous study (Haynes et al., 1997), they treated switchgrass seeds to sodium hypochlorite for 15 min. and seeded. For the short time of exposure, it may not lead negative effects to turfgrass especially for establishment. However, the application of sodium hypochlorite to Kentucky bluegrass during establishment induce negative effects for turfgrass coverage and quality although it had positive effects on algae control. After 46 DAT, no significant differences for all treatments of chemical materials were found and all chemical materials had turfgrass quality greater than six.
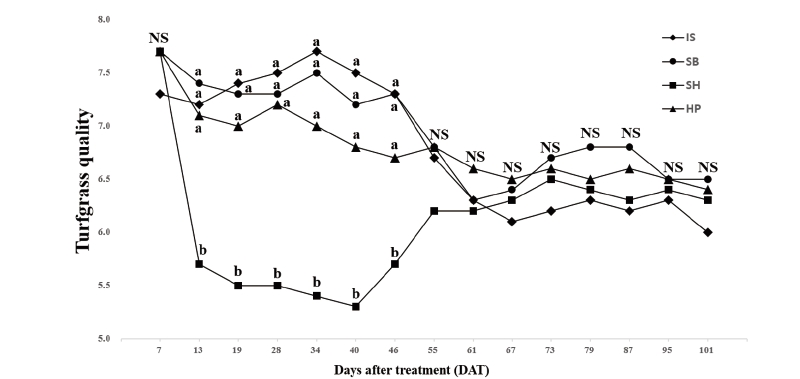
Fig. 3. Mean turfgrass quality per unit surface of ‘Midnight’ Poa pratensis by the chemical material main effect. Each mean was calculated from six observations at four chemical materials (main effect averaged over two application rates with three replications). Means with the same letter within each DAT (days after treatment) or denoted (NS) are not significantly different according to Fisher’s LSD (P =0.05). IS: Iron sulfate (FeSO4); SB: Sodium bicarbonate (NaHCO3); SH: Sodium hypochlorite (NaOCl); HP: Hydrogen peroxide (H2O2).
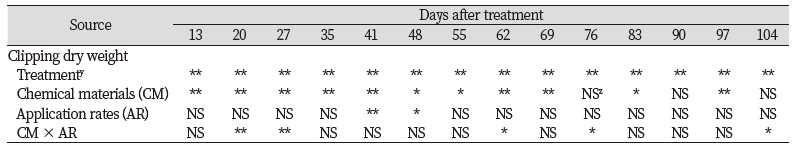
Non-treated control had the greatest clipping dry weight and all chemical materials had negative effects on clipping dry weight compare to non-treated control from 27 to 104 DAT in one-way analysis of variance. There were no consistent significant interaction between chemical materials and application rates for clipping dry weight (Table 5). No significant differences for application rate main effects were found for clipping dry weight on two of 14 rating dates. There were significant chemical material main effects for clipping dry weight on 11 of 14 rating dates. The IS had the greatest or equal to the greatest clipping dry weight for all rating dates while the SH had the lowest or equal to the lowest clipping dry weight (Fig. 4). The IS had 65.6% more clipping dry weight than the SH on 13 DAT. Kentucky bluegrass plot treated by the SH had the greatest reduction of algae coverage with the IS, but it produced the lowest turfgrass coverage, turfgrass quality, and clipping dry weight. In contrast to other chemical materials, the SH had relatively greater negative effects on turfgrass growth. As stated, it may be resulted from the excessive rate of applications or exposure time to chemical materials. However, no research results have been provided about toxicity of sodium hypochlorite to turfgrass growth during establishment.
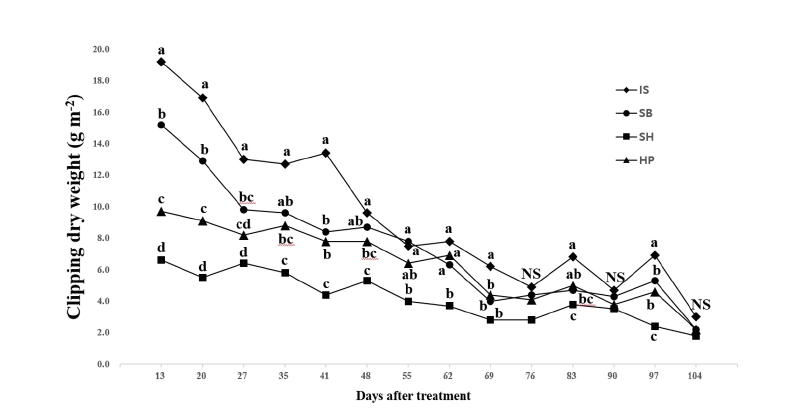
Fig. 4. Mean clipping dry weight (g m-2) per unit surface of ‘Midnight’ Poa pratensis by the chemical material main effect. Each mean was calculated from six observations at four chemical materials (main effect averaged over two application rates with three replications). Means with the same letter within each DAT (days after treatment) or denoted (NS) are not significantly different according to Fisher’s LSD (P =0.05). IS: Iron sulfate (FeSO4); SB: Sodium bicarbonate (NaHCO3); SH: Sodium hypochlorite (NaOCl); HP: Hydrogen peroxide (H2O2).
Overall, the application rates of both low and high rates had no differences for the establishment of Kentucky bluegrass and algae control under the growth chamber study. Iron sulfate and sodium hypochlorite reduced algae coverage at 6.7 and 23.3% during Kentucky bluegrass establishment within 19 days. If the immediate control of algae is necessary, iron sulfate and sodium hypochlorite would be materials to effective algae control based on the results. But, sodium hypochlorite may not be acceptable for Kentucky bluegrass establishment because it had negative effects on turfgrass quality, turfgrass coverage, and clipping dry weight. Further study is required to clarify the reason of negative effects of sodium hypochlorite to turfgrass establishment. Although all chemical materials applied for the study had lower clipping dry weight than non-treated control, iron sulfate had the greatest algae control, turf coverage and turf quality during the establishment of Kentucky bluegrass among all chemical materials used for the study.