Introduction
Chemical pesticides have consistently demonstrated their worth by increasing global agricultural productivity, reducing insect-borne endemic diseases and protecting/restoring plantations, forests, harvested wood products, homes and fiber (Alliance, 2016; Deepak et al., 2013) With the critical need for new crop protection solutions, challenges how to discover the innovative solutions in a timely, cost-effective manner are imperative. Many lead generation strategies across the industry have been employed to find the solutions (Hamaguchi and Hirooka, 2012; Liu et al., 2016). Researches on chemical pesticides have become diverse and complex. Interdisciplinary research utilizing natural product models (Gerwick and Sparks, 2014; Sparks et al., 2017), biochemical design (Drews et al., 2012) and biorational approaches are promising for the discovery of better pesticides (Duke, 2012; Loso et al., 2017; Sparks and Lorsbach, 2017). A wide variety of approaches have been attempted to generate new leads. In the past, pesticides were relatively simple molecules based on their effects on target organisms, but understanding on biochemical processes at cellular level have led to devise relatively complex molecules to selectively block specific metabolic steps. Many are modified forms of natural biocides such as pyrethrins which are chiral, i.e., they have a non-superposable mirror image (‘left-handed’ and ‘right handed’). Usually, only one form (called an enantiomer) is biologically active.
Due to the growing concerns on climate change and sustainability of petrochemical resources, the US Department of Energy announced the selected top 12 biomass-based building blocks and emphasized the need for developing biorefinery technologies to replace the current petroleum-based industry in 2004 (Choi et al., 2015; Jong et al., 2004; Werpy and Pererson, 2004). With the elucidation of chiral pathways, research in this area will greatly be expanded. The exploitation of more chiral pathways will facilitate the development of single enantiamers and boost the pesticide market and ultimately agriculture. Furthermore, marketing elements that favor the adoption of single enantiomers include a ‘green’ image of the product applied at low dose rates, enhancing efficiency to use and reducing the load to environment. This perspective suggests the ways to produce enantiomerically pure pesticides using emerging technologies. In particular, biomass could be transformed into chiral building blocks through appropriate modifications to form the so-called ‘chiral pool’ for providing enantiomerically enriched sources. Development in conversion technology will open up the practicality of pesticides beyond research.
Chirality and chiral pesticides
Chirality as an organic chemistry was introduced in the 1970s by asymmetric reduction and subsequently in the 1980s by asymmetric oxidation. With these achivement, Knowles, Noyori and Sharpless were awarded the Nobel Prize in Chemistry in 2001 (The Nobel Prize, 2001). Enantiomerically pure compounds have many advantages in efficacy over their racemic forms. Chiral chemicals that could not be obtained from petroleum could be produced by utilizing biomass feedstocks. All proteins, enzymes, amino acids, carbohydrates, nucleosides and a number of alkaloids and hormones are chiral.
Chiral pesticides
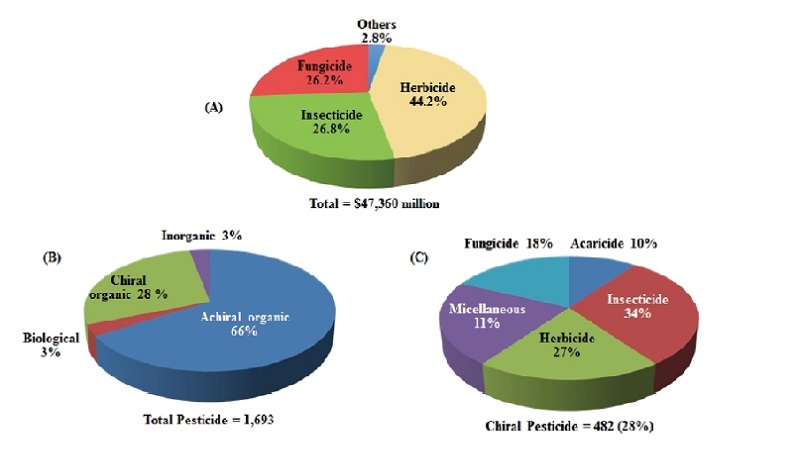
According to the criteria for molecular chirality as defined by the International Union of Pure and Applied Chemistry, the number of chiral pesticides accounts for 28% of all commercial pesticides in the world market (Fig. 1), and this rate has been increasing (Jeschke, 2018; Ulrich et al., 2012). Chiral pesticides usually exhibit some degrees of stereoselectivity in their biodegradation rate and/or toxicity (Albuquerque et al., 2018). Since the majority of the chiral pesticides are prepared and applied as racemic mixtures, their stereoselectivity is needed to be defined for providing improved risk assessment and in some cases for establishing the rationale for the production of single or enriched enantiomeric products (Garrison, 2011; Jeanmart et al., 2016). Considering that pesticides should be developed in a manner of increasing their safety and mitigating various environmental hazards (Ulrich et al., 2012), chiral pesticides might be one strategy to achieve these goals. Stereoisomeric mixtures with multi-chiral centers have recently been increasing as new crop protection agents (Garrison, 2011; Williams, 1997), but their chiral centers were not resolved. With increasing chiral products, unnecessary chiral components in the molecules also increase and thus unintended economic and environmental problems may arise. To develop new chiral pesticides, the biological activity of the molecules as well as their environmental behaviors and fates should be considered (Donate, 2014; Müller and Buser, 1997; Müller and Kohler, 2004; Nguyen et al., 2006; Williams, 1997). Compared to racemates, chiral products using single enantiomers require lower packaging, cargo and storage costs at any stage of the distribution routes (Donate, 2014; Müller and Kohler, 2004).
Chiral herbicides
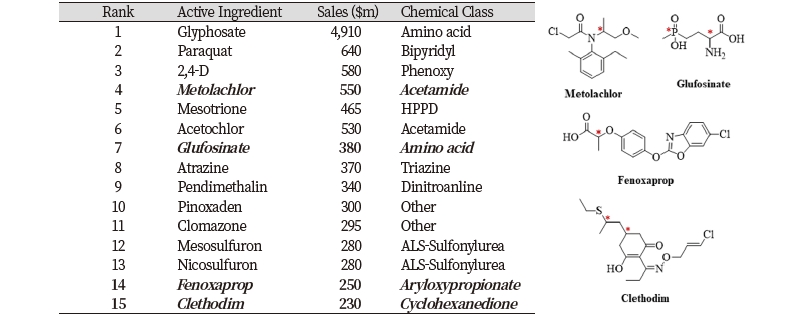
Most compounds of the 141 chiral herbicides, herbicide safeners and plant growth regulators have only one or two chiral centers, but brassinolide and epocholeone have 13 chiral centers. Among the 15 leading herbicides the world market (Phillips McDougall AgriService, 2013), metolachlor, glufosinate, fenoxaprop and clethodim are chiral (Table 1). For example, (R)-form of fenoxaprop is an active enantiomer, while (S)-forms of glufosinate and metolachlor are active. However, these chiral herbicides are difficult to be synthesized, characterized and analyzed. Furthermore, their fates and risks are difficult to be predicted if they are intentionally exposed to the environment.
Chiral insecticides
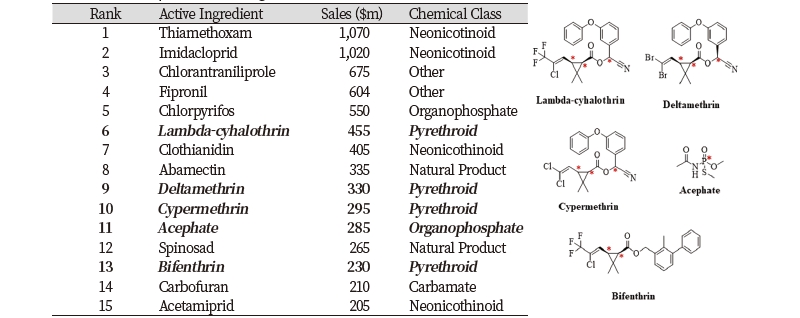
Most of the 149 chiral insecticides have 1-4 chiral centers, but biologically derived pesticides such as abamectin, allosamidin, azadirachtin, emmamectin, sabadilla, spinetoram and spinosad have more than 12 chiral centers. Since many chiral insecticides have ring-constrained chiral features, the number of their stereoisomers is limited. For example, the organochlorine insecticide chlordane has six chiral carbon atoms, but only two of them are unconstrained. Thus, four stereoisomers which are a pair of enantiomers and the trans-chlorides of cis-chlorodon could be made. Various chiral insecticides like pyrethroids have been produced in single or enriched isomeric formulations (Liu et al., 2005). Among the 15 leading insecticides in the world market (Ulrich et al., 2012), λ-cyhalothrin, δ-methrin, cypermethrin, acephate and bifenthrin are chiral (Table 2).
Chiral fungicides
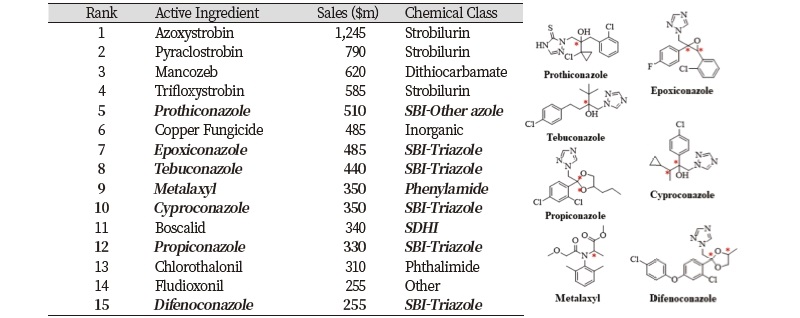
Ninety seven chiral fungicides have 1-4 chiral centers like other pesticides (Liu et al., 2005). In particular, aureofungin, blasticidin-S, cycloheximide, griseofulvin, kasugamycin, natamycin, polyoxin and validamycin antibiotics have several chiral centers. At least four fungicides are currently produced as single or enriched stereoisomeric formulations, which might be applied at lower rates and/or costs. New fungicides having more complex chiral molecules are required to be developed, since resistance to the current fungicides is becoming more diverse and intense (Jeanmart et al., 2016). Of the 15 leading fungicides in the world market, chiral fungicides account for 47% of the total. They include prothiconazole, epoxiconazole, tebuconazole, metalaxyl, cyproconazole, propiconazole and difenoconazole (Table 3).
Chiral building blocks from biomass feedstocks
Chiral synthesis
In the 21st century, with the strengthening of global environmental regulations, the fine chemicals industry was forced to be switched to eco-friendly production processes. Green chemistry has been taken as an alternative to existing chemical reaction processes (Evenson and Gollin, 2003), for minimizing the use of organic solvents, simplifying processes, minimizing byproducts and maximizing intermediate reaction efficiencies. Various technologies have now been available to prepare enantiomerically pure compounds (Kurihara, 2003; Wu et al., 2011). Single enantiomers have been generated traditionally in five main ways, i.e., from a ‘pool’ of existing materials, by classical resolution and associated techniques, by a range of biological methods, by chemically catalyzed asymmetric synthesis and by physical separation methods (Kurihara, 2003; Yu et al., 2016). By contrast, natural products themselves could be used as chiral building blocks in synthesis (Gerwick and Sparks, 2014; Sparks et al., 2017; Wu et al., 2011). Natural products are rich source of enantiomerically pure materials that could be converted into chiral building blocks so called ‘chiral pool’ through appropriate modifications (Donate, 2014; Nguyen et al., 2006; Yu et al., 2016). Although asymmetric synthesis (Christmann and Bräse, 2013; Zhao and Ding, 2013) is still indispensable for obtaining enantiomerically pure compounds, it often requires expensive chiral catalysts and subsequent tedious chiral resolution processes (Izake, 2007, Xie et al., 2008).
Chiral building blocks
Even though petroleum-based chiral chemicals have relatively low chiral purity, their synthesis requires a large amount of organic solvents and energy for introducing additional functional groups, ultimately generating massive amounts of CO2. By contrast, biomass-based chemicals basically contain chiral carbons and thus have advantages in which additional functional groups do not need. Relatively low molecular weight, enantiomerically pure building blocks are required for the synthesis of chiral pesticides. However, single enantiomers are not common (Ikai and Okamoto, 2009), because of limited chiral biomass feedstocks and economic synthesis methods. Most of the chemical products from petroleum are produced in the functionalization direction for adding functional groups such as ethylene, ethylene oxide, n-butane and butadiene, while the biorefinery processes proceed in the de-functionalization direction of hydroxy, carboxy and amino groups. Thus, different approach (‘chiron approach’) from the traditional technology is needed to obtain chiral building blocks from biomass feedstocks. For example, carbohydrates from biomass feedstocks are cheap and abundant in nature, and have rich functionality, chirality and regeneration with high purity (Wu et al., 2011).
Biocompatible chiral organic materials are also important as the core intermediates and raw materials for pharmaceuticals, physiologically active substances such as cosmetics, fragrances and sweeteners, functional food additives, biodegradable bioplastic monomers and diagnostic organic materials (Balaraman et al., 2012; Kabasci, 2014; Rose and Palkovits, 2012). Although the technologies utilizing biomass feedstocks have been limited to the bioenergy sector in the past, the field of platform chemical development utilizing biomass feedstocks creates higher value-added chemicals compared to the energy field (Rose and Palkovits, 2012; Ruppert et al., 2012). For example, alcohols and carbonyl groups can be obtained from cellulose by oxidative cleavage (Beeson et al., 2012; Corne et al., 2013), and useful chiral ligands can be obtained from isohexides (Wu et al., 2011) and aza-sugars (Lichtenthaler, 2002). Selective reactions of functional groups include the changes of carbon skeleton through oxidation/reduction, oxidative cleavage and ring-opening/closing metathesis, as well as interactions of basic functional groups such as esterification, etherification and carbamate formation. Chiral chemical materials including natural/unnatural amino acids, amino alcohols and diamines could widely be utilized for the production of chiral drugs, biologically active materials, functional food additives and biodegradable bioplastic monomers (Kabasci, 2014).
Enantiomerically pure pesticides synthesis from biomass feedstocks
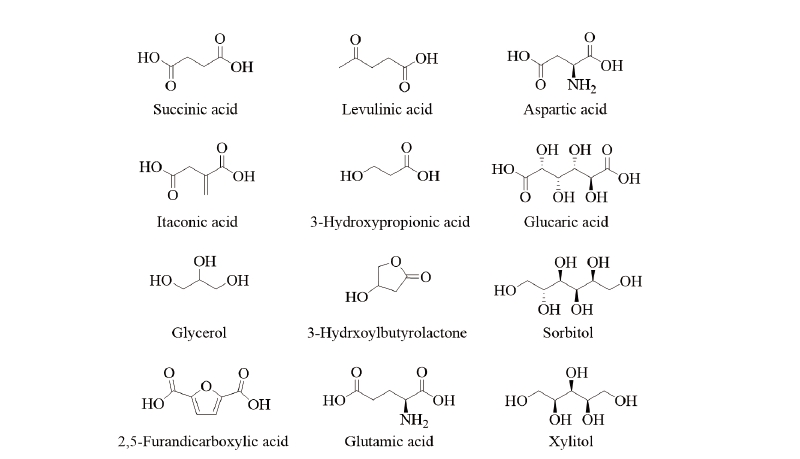
In 2004, the US Department of Energy reported the 12 building block chemicals (Fig. 2) that can be produced with sugar through biological or chemical conversion, and can be consistently converted into a variety of high value-added biomass-based chemicals. The 12 building blocks were selected from a list of over 300 candidate chemicals. Pacific Northwest National Laboratory and National Renewable Energy Laboratory selected the 30 candidate substances using a recursive review process based on petrochemical models, chemical data, known market data, attributes, potential performance and previous industry experience. From this list, they reviewed the potential market size of the derivative products and the technical complexity of the synthesis routes, and ultimately reduced the list to 12 chemicals with their building blocks. They include gluconic acid, lactic acid, malonic acid, propionic acid, citric acid, aconitic acid, xylonic acid, acetoin, furfural, levoglucosan, lysine, serine and threonine. Various pathways have been proposed to produce the chiral building blocks from biomass feedstocks (Duke, 2012; Gao et al., 2009).
Chiral building blocks from aspartic acid and lactic acid
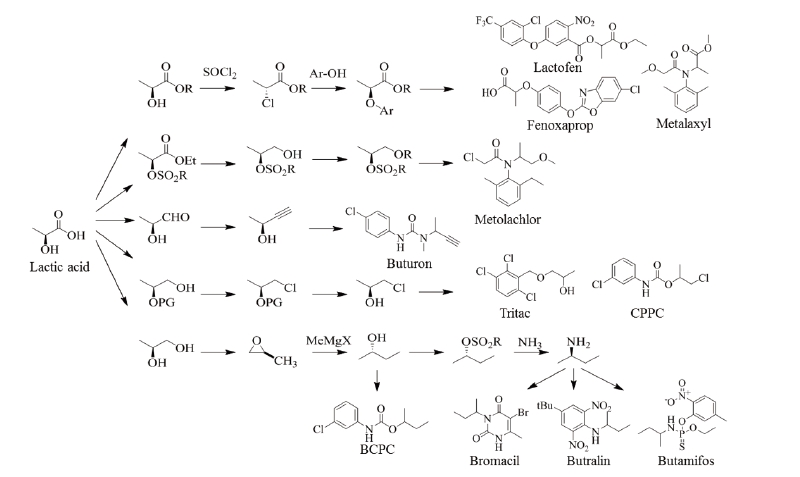
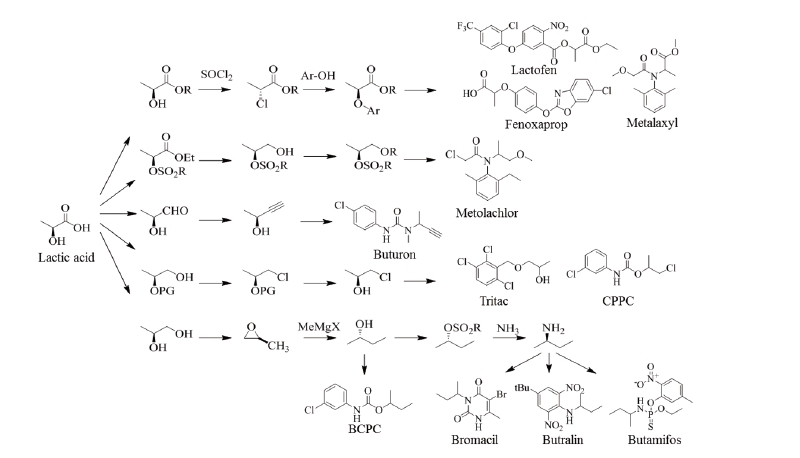
Aspartic acid could be used to produce various chemical analogs such as 1,4-butanediol, tetrahydrofuran and γ-butyrolactone. These analogs have great potential in market polymers and solvent applications. Biodegradable special polymers such as polyaspartic acid and polyaspartates could be synthesized for substituting polyacrylic acid and polycarboxylates. The polymerization will be similar to the commercial process for polyglutamic acid (Bajaj and Singhal, 2011). Aspartic acid could also be utilized as a chiral building block for pesticide synthesis (Fig. 3), but these processes are largely dependent on the large-scale production of aspartic acid. Pesticides such as glufosinate, malathion and CGA 80,000 possess a skeletal aspartic acid.
Lactic acid could be produced from biomass feedstocks like corn and sugar cane and its productivity could be enhanced by utilizing metabolically engineered strains with combining recycling and continuous fermentation technologies. Lactic acid is annually produced in a quantity of 450,000 MT in the world market by the conventional biotechnological processes through the fermentation of carbohydrates using glucose and sucrose as the main starting materials (Gao et al., 2009; Lee et al., 2011). Currently, lactic acid can be produced from the direct formation of methyl lactate of common sugars. Lewis acidic zeotypes, such as Sn-β, catalyze the conversion of mono- and disaccharides dissolved in methanol to methyl lactate at 160℃. With sucrose as the substrate, the methyl lactate yields up to 68%, and the heterogeneous catalysts can easily be recovered by filtration and reused multiple times after calcination without any substantial changes in the product selectivity (Holm et al., 2010). Polylactide, a biomass-derived biopolymer, is a starting material for the production of lactic acid through fermentation processes. Many companies have developed various processing technologies for producing L-polylactide from glucose (Gao et al., 2009), but more economic technologies are still needed to be developed. A significant number of chiral active ingredients contain a propionate backbone, which can be produced starting with (R)- or (S)-lactate esters. Representative examples are shown in Fig. 4.
Chiral building blocks from levulinic acid
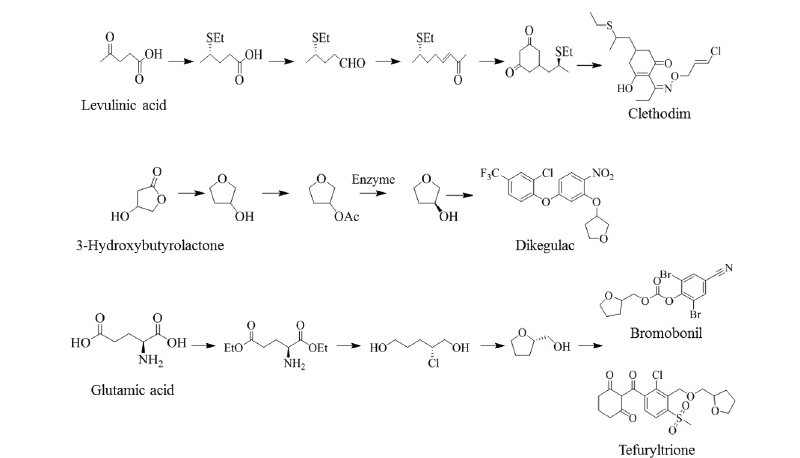
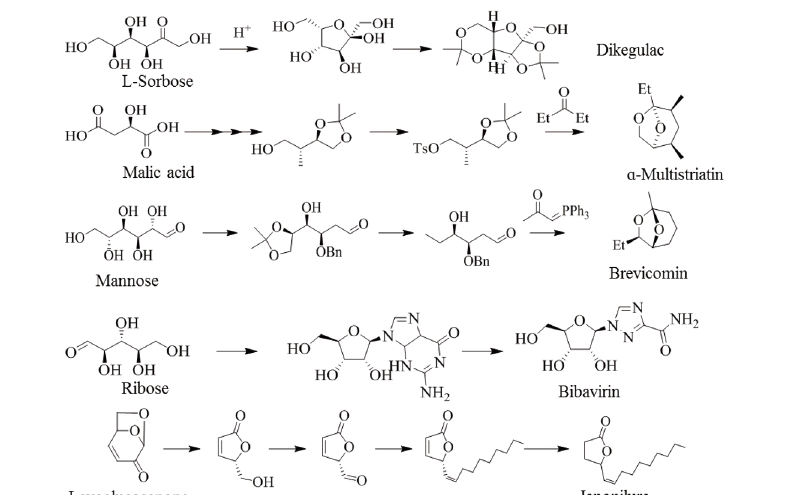
Levulinic acid is produced from starch or lignocellulosic 6-carbon sugars by acid treatment. Five-carbon sugars derived from hemicellulose, i.e., xylose and arabinose, can also be converted to levulinic acid by acid treatment followed by reduction treatment. Thus, levulinic acid could serve as a valuable building block that can be obtained from almost all carbohydrates produced in a biorefinery. From levulinic acid, various derivatives such as 2-methyl-tetrahydrofuran, acrylic acid, δ-aminolevulinate, diphenolic acid, β-acetylacrylic acid, angelilactones and γ-valerolactone can be produced and for that reason levulinic acid has often been suggested as a building block for a wide number of derivatives (Fig. 5). The group of compounds that can be produced from levulinic acid are quite broad and cover many large-scale chemical markets. In addition, δ-aminolevulinic acid, a levulinic acid derivative, has been used as an herbicide and has a market of $ 400-900 million annually. However, further R&D is still required to achieve the optimal productivity. Fig. 5 shows the expected chiral building blocks from levulinic acid for the synthesis of the herbicide clethodim. 3-Hydroxybutyrolactone is a cyclic 4-carbon compound produced through chemical modifications. Research and market opportunities for 3-hydroxybutyrolactone are from their potential to develop new derivatives. Since it is produced as a special chemical for very high value applications, there is little interest in commercial chemical intermediates and all its related development issues. An engineering analysis is needed to better define the metrics in order to achieve the cost-effective production of this molecule (Lee and Park, 2009). Fig. 5 shows the chemistry of 3-hydroxybutyrolactone for the major products of derivatives as well as the expected synthesis route for dikegulac pesticides. For the production of glutamic acid derivatives, the ability to perform selective dehydrogenation in the presence of other functional groups or to convert acidic residues to alcohols in the presence of amines should be considered. Biomass-based products for the 5-carbon building block, glutamic acid, have important market opportunity (Kabasci, 2014). Chiral building blocks could be expected from glutamic acid, and bromobonil and tefuryltrione could be synthesized from the building blocks (Fig. 5).Chiral building blocks from glycerol
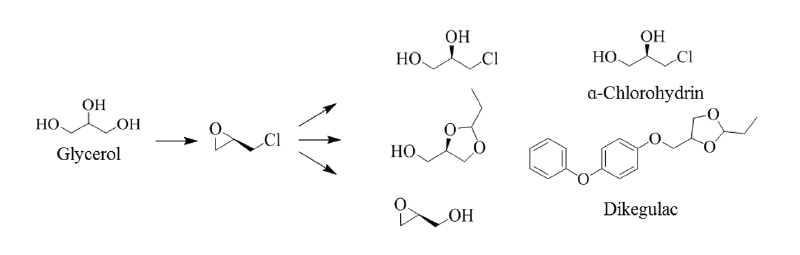
Glycerol is currently a well-known commodity with world production of 500-750 MT per year. Approximately 75% of the US glycerol supply comes from natural resources. Since glycerol is a key coproduct of biodiesel manufacturing, increasing the use of biodiesel will lead to much greater glycerol availability and lower costs. If prices drop to the 0.2 to $ 0.5/lb range, glycerol could become a major building block for biorefinery industry. Nontoxic, edible and biodegradable nature of glycerol will provide significant environmental benefits for new platform products.
Glycerol could be utilized in producing many derivatives owing to its multifunctional structure (Fig. 6). Since glycerol is structurally similar to sugar, the conversion processes developed for glycerol could also be applied to inexpensive sugars like glucose and xylose, greatly diversifying the biorefinery process. The pesticides dikegulac and α-chlorohydrin can be synthesized from the chiral building blocks derived from glycerol (Fig. 6). Other expected chiral building blocks for pesticide synthesis were also presented in Fig. 7. As the chemical structures of new pesticides become larger or more complex with multiple chirality to control new diseases, pests and weed species efficiently, and to meet more favorable environmental and toxicological requirements, natural products and their derivatives will have to drive the development of the new pesticides. However, economic synthesis routes and technologies are currently limited for developing new chiral pesticides from biomass feedstocks. Biorefinery technologies utilizing biomass feedstocks instead of petroleum have now become a subject of interest worldwide and various technologies have been available for producing bio-platform chemicals. Thus, chiral compounds from biomass feedstocks could be used for producing enantiomerically pure pesticides (Schuur et al., 2011; Paik et al., 2009). Chiral building blocks and their derivatives could be used to various ways during the development of new chiral pesticides and the biorefinery technology will accelerate the development. The use of chiral synthons derived from biomass feedstocks could also be expected to reduce CO2 to cope with climate change.
Authors Information
Hyun Seok Yeom, Korea Research Institute of Chemical Technology, Senior Research Scientist
Young Kwan Ko, Korea Research Institute of Chemical Technology, Principal Research Scientist
Hee Jae Lee, Seoul National University, Professor
Hwang In Taek, Korea Research Institute of Chemical Technology, Research Fellow, https://orcid.org/0000-0001-7630-0737