Introduction
Genus Lamium L. belongs to the Lamiaceae family, Lamioideae subfamily and represent both cleistogamous and chasmogamous floral traits (Lord, 1982a, b; Sajjadi and Ghannadi, 2012). Lamium amplexicaule is sensitive to light and could germinate well under dark (Jones and Bailey, 1956). However, Baskin and Baskin (1981) reported the diverse nature of L. amplexicaule seed germination according to the seasonal variation, where, higher germination was observed under light in autumn and dark in winter.
The plant possesses significant pharmacological and biological activities including free radical scavenging, antioxidant, anti-inflammatory, antiproliferative, antinociceptive, antispasmodic cytotoxic, bacteriostatic, and tyrosine inhibitory activities (Sajjadi and Ghannadi, 2012). Lamium amplexicaule is widely reported for isolation of iridoids (Kobayashi et al., 1986), flavonoids and several other antioxidant compounds (Nugroho et al., 2009; Yumrutas and Saygideger, 2010). However, L. amplexicaule serves as a reservoir host of soybean cyst nematode (Creech et al., 2007a; Creech et al., 2007b), and tomato yellow leaf curl virus in Korea (Kil et al., 2014). Moreover, L. amplexicaule is reported as aggressive invasive pest in cropland, pastures and gardens and is reported for yield loss in crops (Conley and Bradley, 2005; Jones et al., 2012). Invasive species such as weeds protect the environment form erosion and nutrients in winter and increases the ecological diversity, however they can serve as pathogenic host (Hill et al., 2014). Moreover, fertilizer application has significant effect on L. amplexicaule adaptation. It has been reported that henbit biomass was 28% greater when applying N in the autumn compared to an early preplant application timing Nelson (2015). Nitrogen placement along with associated tillage with strip-till placement was important in reducing henbit biomass (Nelson, 2015). Hence, relevant management of L. amplexicaule is crucial for disease management and optimum agriculture production.
Herbicides comprise nearly 40% pesticides used over the world. Herbicides function through binding metal such as magnesium, calcium, and manganese that subsequently inhibits the biosynthesis of aromatic amines necessary for plants and microorganisms (Turkmen and Dogan, 2020). Herbicides such as glyphosate application could control henbit plant and hence reduced soybean cyst nematode infestation (Werle et al., 2013). Glyphosate (N-(phosphonomethyl) glycine) is a systemic, non-selective, post-emergence herbicide that controls weeds in a wide range of both non-agricultural and agricultural land (Travlos et al., 2017). In addition, glyphosate has been considered as non-persistent in the environment and does not pose a health risk to human (Solomon and Thompson, 2003; Williams et al., 2000). However, Fu et al. (2020) showed the probable evidence of glyphosate toxicity in liver of piglets. Similarly, glufosinate is reported for several abnormalities in mice such as disturbed gut microbiome and neuro development (Dong et al., 2020). Glufosinate is an alternate herbicide to manage glyphosate resistant weeds. It disrupts photorespiration leading to photoreduction of oxygen molecules and generates reactive oxygen species (ROS). There is controversial opinion about the glufosinate toxicology, however, appropriate use can be considered safe (Takano and Dayan, 2020).
Management of invasive weed species plays a key role for sustainable agriculture production (Park et al., 2020). The international trade has been the major source of invasive weed invasion globally and has increased the threat to ecology, environment, livestock including mankind (Kim et al., 2020). Thus, huge investments are disseminated for control and management of invasive weeds (Clements et al., 2004). In addition, the excessive anthropogenic activities and use of agrichemicals is imposing toxicity in organisms through stimulating the flow of heavy metals in urban soil (Jiang et al., 2018). In these scenarios, minimal doses of agrochemicals that results maximum use efficiency is the ideal option for effective management of weed. Hence, the objective of this study is a baseline to identify the appropriate doses of herbicides to gain optimum control rate against L. amplexicaule and to elucidate the effect of herbicides on its growth characteristics.
Materials and Methods
Leaf disc bioassay
The in-vitro experiment was carried out to examine the effects of herbicide concentration on the henbit deadnettle color disintegration in leaves. Here, equal size leaves were placed on a filter paper placed in a inner disc of a petri dish (60×15 mm) and treated with herbicides solution. The plates were sealed to prevent contamination and kept in growth chamber at 25℃. Please descript what authors observed or measured such as color.
Pot experiment and non-selective herbicides application
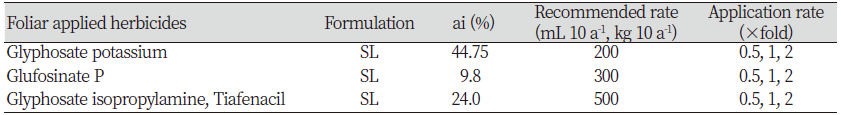
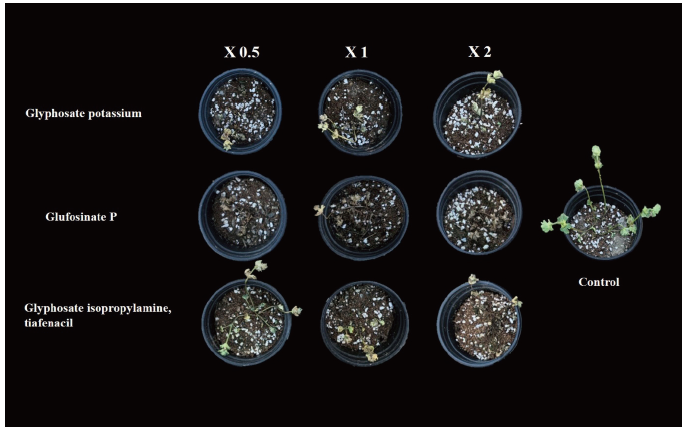
To investigate the management of henbit deadnettle by herbicide treatments of the foliage, 32 seeds were sowed in a plastic tray (32 holes) After 30 days, six equal size of plants were selected and transplanted to pot (100 mm×90 mm). Ten days after transplantation, the pots were treated with a different herbicide with one-half, one and two-folds of recommended rate. In brief, approximately 10 mL pot-1 of herbicides prepared according to the application rate (0.5, 1, 2) of recommended dose was applied using and sanitizing 200 mL bottle sprayer (Table 1). After 21 days of herbicide treatment, fresh weight and dry weight were measured, and control values were calculated by following formula.
Control value (%)=(untreated group weight-treated group weight/untreated group weight)×00
Measurement of chlorophyll content
The chlorophyll content was measured according to the method described by (Lichtenthaler and Wellburn, 1983). In brief, the absorbance was measured at 663, 646, and 646 nm by using T60 UV-Vis Spectrophotometer (PG instruments Ltd., Wibtoft, UK). The content of chlorophyll a and chlorophyll b were calculated by using the following formula:
Chlorophyll a=12.21 (663)-281 (646)
Chlorophyll b=20.13 (646)-5.03 (663)
Statistical Analysis
The experiment was conducted in a complete randomized design. Statistical analysis was performed with SAS 9.4 software (SAS Institute, Cary, NC, USA) and the mean values among the treatments were separated using Duncan’s multiple range test. Each treatment has six replicates and the experiment was repeated three times.
Results
Analysis of leaf-disc bioassay
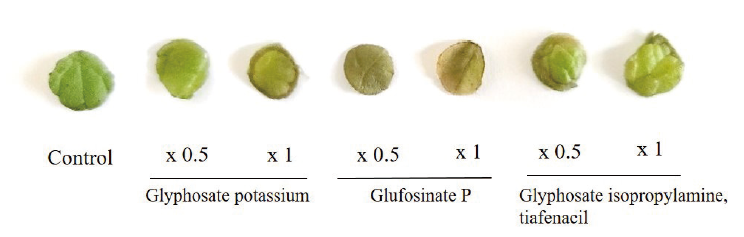
The leaf disc bioassay study revealed that the glufosinate P rapidly degenerate the pigment when compared to the other. Moreover, glyphosaate potassium one-fold application has considerably higher color disintegration as compared to glyphosate isopropylamine+tiafenacil (Fig. 1).
Effect of herbicides on chlorophyll content of L. amplexicaule leaves
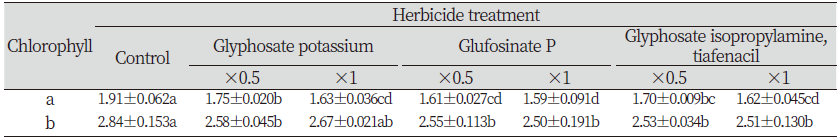
The current research revealed that the chlorophyll content of L. amplexicaule were significantly lowered by the application of herbicides. The chlorophyll a was significantly reduced with all the herbicides treatments whereas considerable reduction was observed on chlorophyll b. Among them, glufosinate P application has significant effect on lowering both the chlorophyll a and b compared to other herbicides (Table 2).
Effect of herbicides on morphological growth attributes of L. amplexicaule plants
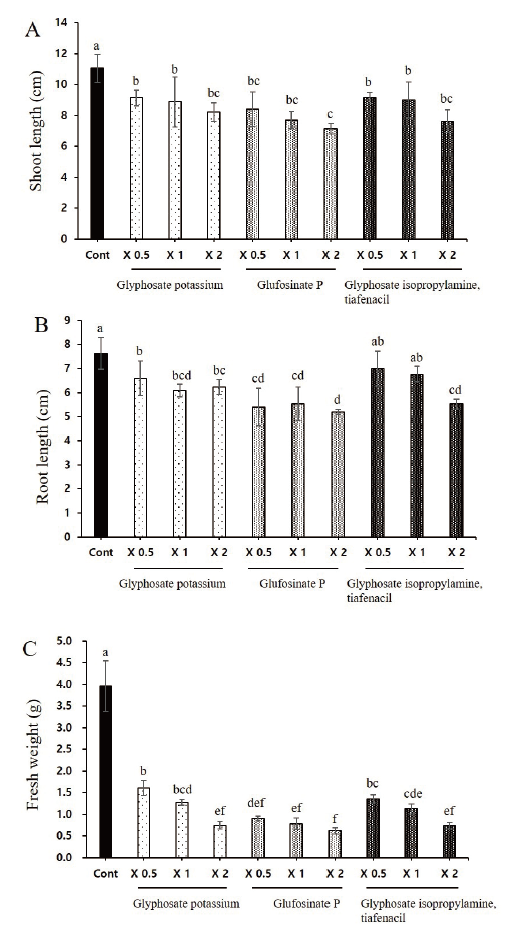
The herbicides application significantly lowered the plant growth promoting characteristics such as root length, shoot length and total biomass of L. amplexicaule plants. The plant height of deadnettle significantly reduced with the application of all three herbicides in which two folds application of glufosinate P had significantly lowered the shoot length. Similar trend was observed on root length and fresh weight (Fig. 2).
amplexicaule plants. Each data point represents the mean of at least six replications. Vertical bars with different letters are significantly different from each other at P≤0.05.
Effect of herbicides on the control rate or L. amplexicaule plants.
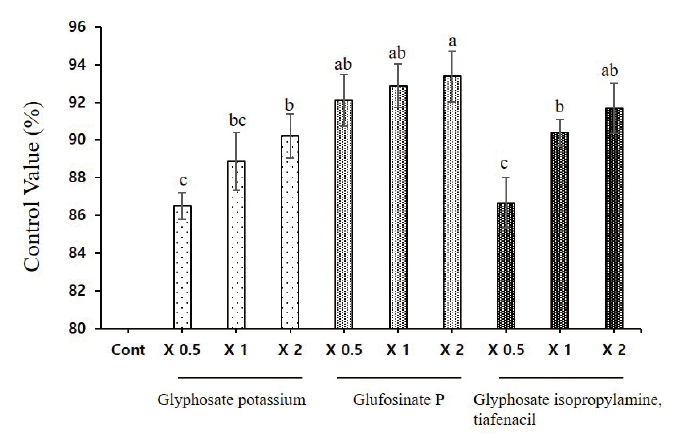
The current results showed more than 90% control rate with the treatment of glufosinate P even with one-half dose application. The differences between the on-half and two-fold application of glufosinate P on control rate differs only with 3%. The glufosinate P with two-fold application has the highest control rate over henbit deadnettle. In addition, the one-half dose application of glufosinate P is sufficient to achieve the maximum control rate as compared to the two fold application of glyphosate potassium and glyphosate isopropylamine tiafenacil. Moreover, no significant differences were observed on the control rate when compared to glyphosate potassium with glyphosate isopropylamine tiafenacil (Fig. 3 and Fig. 4).
Discussion
Invasive weeds can be toxic to livestock and detrimental to agriculture production. Hence, chemical pesticides such as glyphosate and glufosinate are widely used to control the weeds to protect the environment and agriculture from possible destruction. Since, herbicides interfere the metabolism that led to phyto-toxicity and ultimately inhibits the growth and development of plant, phyto-toxicity on crops should be considered prior to herbicides application (Grichar et al., 1996). Here we discuss the possible effect on application with glyphosate and glufosinate with different doses to control L. amplexicaule.
Effect of herbicides glyphosate potassium, glufosinate P, and glyphosate isopropylamine with tiafenacil as foliar application was examined on L. amplexicaule. The results showed that the application of glufosinate P with two-fold application has significant effect to control L. amplexicaule with 94%. Here, the control rate of glyphosate isopropylamine with tiafencail has minimum control rate. Interestingly, the control rate of one-half dose application of glufosinate P has a greater control rate as compared to that of two-fold application of either glyphosate potassium or glyphosate isopropylamine with tiafencail. Similar results were shown by Grey et al. (2006) who showed the application of glufosinate 0.5 and 1.0 kg a.i. ha-1 and glyphosate 0.84 and 1.64 kg a.e. ha-1 application led to 75% control rate. Other herbicides such as imazamox, oxyfluorfen flumioxazin and rimsulfuron: thifensulfuron, have considerable effect to control L. amplexicaule (Grey et al., 2006; Woolam, 2016).
The mode of action of herbicides influences the efficacy, severity, and speed of damage against the target (Park et al., 2018). Here, glyphosate also known as 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) inhibitor is the most used (by land area and volume) herbicides in the world (Duke and Stephen, 2008). Low dose of glyphosate can cause hormesis due the increase in photosynthetic rate associated with high efficiency of CO2 fixation (Cedergreen and Charlotte, 2010). Extensive use of glyphosate can impose nutrient deficiency but little is known about potassium (K) interactions with glyphosate (Lane et al., 2012a). The fertilizer works through inhibiting the enzyme EPSPS (Kremer and Nathan, 2009). This enzyme catalyzes the formation of EPSP, a critical intermediate, used in the production of three aromatic amino acids vital to plants (Lane et al., 2012b). Moreover, glyphosate application can severely affect the microorganism since it hinders the shikimic acid pathway on fungi and bacteria (Busse et al., 2001). Long-term effect of glyphosate may increase K deficiency in plants (Lane et al., 2012b).
Similarly, glufosinate (2-amino-4-(hydroxymethylphosphinyl) butyric acid) is a synthetic isomeric mixture of d,l-phosphinothricin, a microbial phytotoxin discovered in bacteria of the genus Streptomyces (Takano et al., 2020). Glufosinate function as glutamine synthase inhibitor that inhibits glutamine synthesis through assimilation of toxic ammonia incoroporating into glutamate to yield glutamine, whichdisrupts amino acid synthesis and alter the chlorophyll fluorescence (Dayan and Zaccaro, 2012; McNally et al., 1983). Glufosinate concentration in leaves is proportional to GS inhibition, ROS accumulation, and phyto-toxicity (Takano et al., 2020). Tiafenacil causes protoporphyrin IX accumulation and oxidative damage to plants (Park et al., 2018).
Herbicides application inhibits photosystem, carotenoid synthesis hence generates a reactive oxygen species and lipid peroxidation and finally interfere with the modes of action of photosynthesis (Dayan and Zaccaro, 2012). These symptoms corroborate to our results as the disruption of photosynthetic activity through degrading the chlorophyll pigment is observed on herbicides application on L. amplexicaule plants.
Conclusion
Overall, the herbicide glufosinate functioned well against disrupting the photosynthetic activity as signified by the rapid degradation of chlorophyll content and 94% control rate the current research recommends the application of glufosinate to attain maximum outcome against L. amplexicaule incidence and distribution.
Acknowledgement
This study was supported by the Agenda Program (Project No. PJ015026022021) Rural Development Administration, Republic of Korea.
Authors Information
Eun-Jung Park, Department of Applied Biosciences, Kyungpook National University, Researcher
Arjun Adhikari, Department of Applied Biosciences, Kyungpook National University, Doctor of Philosophy
Lee-Rang Kim, Department of Applied Biosciences, Kyungpook National University, Master student
Eun-Hae Kwon, Department of Applied Biosciences, Kyungpook National University, Master student
Ki-Yong Kim, National Institute of Animal Science, RDA, Researcher
In-Jung Lee, http://orcid.org/0000-0001-7154-4820