Natural introduction and accidental introduction of exotic weed occurs through the movement of winds (Benvenuti, 2007). Moreover, massive industrialization has influenced the exposure of exotic plants through climate change and the expansion of international trade (Adebayo and Uyi, 2010; Oh et al., 2002). Exotic plants may have no effect or minor effect but sometimes may become invasive and show significant effect on the bio-diversity and ecological system (Clements and Ditommaso, 2011; Kim et al., 2015; Kim et al., 2017). In agriculture, exotic weeds colonization has enhanced serious loss in grain production and have been reported for the global threat for food security (Orapa, 2001). In livestock, exotic weeds can result in severe diseases when consumed by livestock in hay or pasture, if it contains toxic (Puschner, 2017).
In Korea, the influx of exotic plant is rising rapidly. It has been reported that at least 158 annual weed species among which 100 species were inflowed within the last one decade. These inflows comprise 42% from Europe, 23% from North America, 9% from Eurasia and 8% from tropical America (Kim et al., 2000; Ko et al., 2019).
Rumex obtusifolius is a broad-leaved dock, native to Europe. It was introduced in Korea before 1994 and spread out almost everywhere in Korea (Jung et al., 2017). R. obtusifolius is a major weed in gardens and arable land (Holm et al., 1977; PIER, 2007). The GCW (2007) listed the R. obtusifolius as an agricultural and environmental weed. On agricultural land it commonly grows in meadows and pastures, abandoned fields, field borders, hedgerows and orchards. It also occurs as a ruderal on roadsides, ditch banks, and along riversides and streams, and can be found in woodland margins and forest clearings (CABI, 2018). Broad-leaved dock is a pernicious weed throughout its native and introduced range. It invades agricultural land, particularly heavily managed pasture land. Seeds and vegetation of docks can be toxic to animals because contains oxalic acid (Royer and Dickinson, 1999).
Due to its ecological effects and global consequences, considerable attention has been given to exotic weeds in recent years (Bai, 2014). An understanding about biology, ecology and germination characteristics is a necessary first step to achieve complete control. Likewise, in order to effectively manage rapidly spreading weeds, we need to know how weeds occur first (Lee et al., 2017). Therefore, the objectives of this study were to determine the effect of different environmental factors on seed germination and seedling emergence of R. obtusifolius.
Dormancy of Rumex obtusifolius seeds after harvest
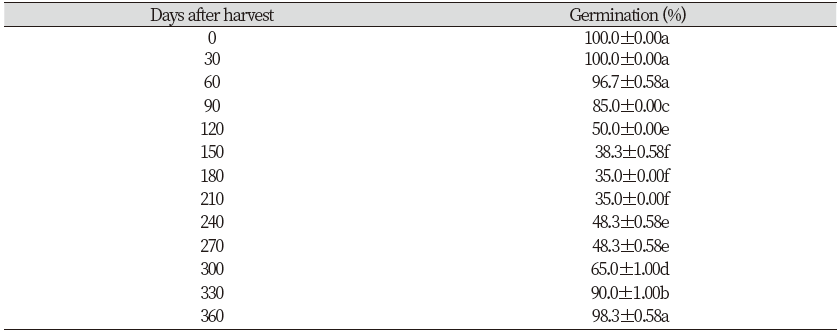
To study the effects of after-ripening time, seed germination tests were carried out at 0 to 360 days after harvest. Tests were installed with three replications of twenty seeds per Petri dishes (60×15 mm), two filter papers and moistened with 5 mL of distilled water. After that, it was incubated for 15 days in 16/8 h light/dark condition at a room temperature (20-25℃). Water was replenished as needed. Germinated seeds were counted and removed every 24 h throughout the experiment. Finally, the total of germinated seeds were calculated.
Results illustrated that the germination percentage of seeds of R. obtusifolius was reached to 96.7% (up to 100%) during first 2 months after harvest. After that, seeds exhibited a degree of dormancy at 150 to 210 days after harvest where the seed germination was decreased. The dormancy was progressively lost after several months of storage at room temperature; results indicated that the seed germination was almost 90% after storage 330 days after harvest (Table 1).
Germination by temperature
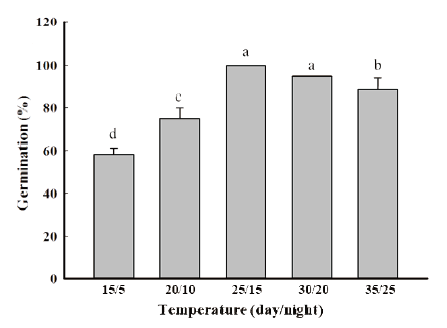
Twenty seeds were placed on a Petri-dish (60×15 mm) with two layers of filter paper and 5 mL of distilled water was added. Then it was placed into a Growth Chamber under 16/8 h (day/night) photoperiod. Germination temperatures were 15, 20, 25, 30 & 35℃ and 5, 10, 15, 20 & 25℃, at day and night time respectively. There were three replications, and the final germination was determined at seven days after sowing (DAS).
Temperature fluctuation was also found to act as a strong germination trigger for germination. R. obtusifolius seeds were reported to give maximum germination in light at 25/15℃ to 30/20℃ (day/night). Many studies agreed that ideal temperature condition for the seed germination of R. obtusifolius in the growth chamber is 25℃ (Benvenuti et al., 2001; Khalid, 2018). The germination was below 60% at 15/5℃ (day/night) and it will increase with rising temperatures. However, at high temperature above optimal temperature limits the seed germination as a result of the damage the seeds. Our results are in line with Cavers and Harper (1964) who reported the seeds can germinate throughout the year but most are found in the spring and in the autumn (Fig. 1).
Salt stress
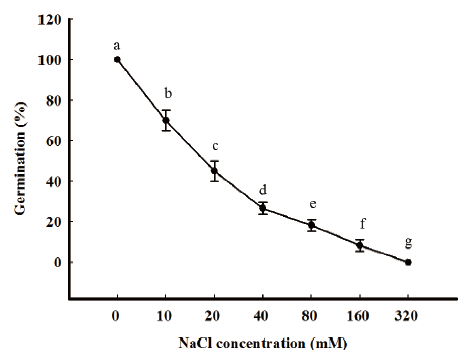
To investigate the effect of salinity on the germination of seeds, different concentrations of NaCl solutions (0, 10, 20, 40, 80, 160, and 320 mM) were used in the germination test. Twenty seeds were incubated on two layers of filter paper in a Petri dish (60×15 mm), to which 5 mL of each NaCl solutions were added. For each of these trials, the treatment with sterilized distilled water was included as a control. All petri dishes were placed in an illuminated incubator subjected to a 16h daily photoperiod at 25/15℃ (day/night). The seed germination was determined at seven DAS.
In general, germination was significantly affected by salinity. Our preliminary results showed that increased salinity results in decreased germinability and delayed rate of germination. At the salinity of 10 mM, the germination was 70% and seed germination percentage did not reach 30% at salinities exceeding 20 mM. R. obtusifolius germinated in very low percentage (under 10 %) at 160 mM salinities and no seeds germinated at salinities greater than 320 mM (Fig. 2).
The relative growth of plants in the presence of salinity is termed their salt tolerance. A high salt level interferes with the germination of seeds. Published data shows that the effect of NaCl on seed germination is mostly osmotic (Loercher, 1974; Reynolds, 1975). It can be concluded that salt reduces the water potential of soil solution, which prevents the water supply and proper nutrient balance in plants. Although salinity stress mostly reduces the germination percentage and delays the onset of germination, seeds have stayed viable at soil and being able to germinate under appropriate external conditions such as temperature and light (Khan and Ungar, 1997). Seed germination takes place after high precipitation, i.e., under conditions of reduced soil salinity (Khan and Rizvi, 1994), rain dissolves and washes away salt deposits and provides enough water for germination. That’s why, R. obtusifolius is considered as one of the most widely distributed weeds in the world.
Emergence by burial depth
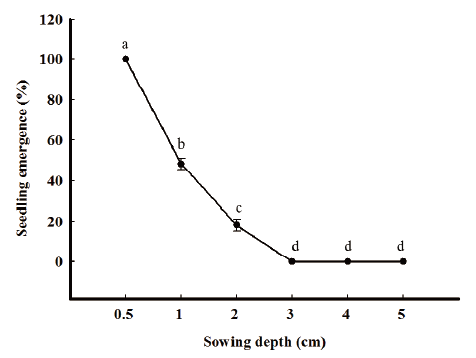
Ten seeds were sown into a plastic pot (size: 7 cm diameter, height 10 cm) filled with mixed soil (1:1 [w/w]) of horticultural nursery soil: rice nursery soil. Burial depths were 0.5, 1, 2, 3 and 4 cm and replications were three. At 21 DAS, the number of seedlings was measured. Plastic pots were placed in a greenhouse subjected to a 16h daily photoperiod at 20-25/10-15℃ (day/night).
R. obtusifolius seedling emergence was strongly inhibited by burial depth. The germination was declined with increasing depth of soil cover. The germination of R. obtusifolius was significantly higher at 0.5 cm (100%) as compared to other sowing depths. Shallow burial, even at a 1 cm depth, halved reduced the percentage seedling emergence of R. obtusifolius. The threshold depth level for seedling emergence was considered 2 cm as none of the seedling emergence was observed at 3 cm depth and deeper (Fig. 3). Our results are similar to Van Assche and Vanlerberghe (1989), Weaver and Cavers (1979) who have reported the burial depth effects on R. obtusifolius seedling emergence.
Based on these results, it can be concluded that the R. obtusifolius seeds thrive better at 25/15℃ to 30/20℃ (day/night) and more successful in salt-free settings or in those having extremely low saline conditions (20 mM of NaCl). Until 60 days after harvest, R. obtusifolius seeds were non-dormancy, then exhibited a degree of dormancy at 150 to 210 days after harvest. After long time storage at room temperature, the dormancy was progressively lost and got reaching full germination (330 days after harvest). The sowing depth of 0.5 cm is considered optimum for R. obtusifolius emergence. These results will be useful to develop integrated exotic weed management in the R. obtusifolius infested area in Korea.
Acknowledgements
This work was carried out with the support of the “Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ016128012021)” Rural Development Administration, Republic of Korea.
Authors Information
Thi Hien Le, Chungnam National University, Ph.D. student
Sug-Won Roh, Rural Development Administration, Doctor of Philosophy
Mirjalol Umurzokov, Chungnam National University, Ph.D. student
Aung Bo Bo, Chungnam National University, Ph.D. student
Weiqiang Jia, Chungnam National University, Doctor of Philosophy
Kwang Min Cho, Daeseung Bio Farm Research Center, Doctor of Philosophy
Kee Woong Park, Chungnam National University, Professor