Introduction
The constant use of the same herbicide or herbicides that affect a single target area leads to a selection of herbicide-resistant weed populations. Resistance to acetolactate synthase (ALS) inhibitors was first recorded in 1998 in Monochoria korsakowii in Korea (Park et al., 1999). Since then, 15 weed species were confirmed resistant to different herbicides, which is nearly 20% of the total weed species occurring in rice fields (Bo et al., 2017; Bo et al., 2019; Lee et al., 2017b). A survey in 2011 showed that 72,736 ha of rice fields in Chungcheongnam-do, 35,194 ha in Jeollabuk-do, and 21,410 ha in Jeollanam-do were infested with herbicide-resistant weeds (Lee et al., 2012). Another survey in 2017 reported that the area infested with herbicide-resistant weeds in Chungcheongnam-do, Jeollabuk-do, and Jeollanam-do provinces increased up to 85,978, 81,494, and 91,543 ha respectively (Jeong et al., 2018; Won et al., 2018). The occurrence and distribution of herbicide-resistant weed species were investigated (Choi et al., 2018; Han et al., 2019; Jeong et al., 2018; Lee et al., 2017a; Lee et al., 2019a; Lee et al., 2019b) and reported that the incidence of herbicide-resistant weeds is significant in Korea, and if this continues, we expect more affected areas in the future.
Scirpus juncoides is the third most noxious weed after Echinochloa spp and Monochoria vaginalis in the paddy field of Korea (Lee et al., 2017b). A high density of herbicide-resistant S. juncoides resulted in up to 40% loss of rice yield (Kuk et al., 2002; Sada et al., 2013). The prevalence of herbicide-resistant S. juncoides in paddy fields has increased from 7,032 ha in 2012 to 14,963 ha in 2018 in Chungcheongnam-do province of Korea (Lee et al., 2012; Won et al., 2018).The excessive and unreasonable use of herbicides that inhibit acetolactate synthase (ALS) has led to the evolution of ALS inhibitor-resistant S. juncoides. In Korea, S. juncoides first evolved resistance to ALS inhibiting herbicides in 2001. Research has confirmed that Jeollabuk-do, Kimjea accession was resistant and cross-resistant to sulfonylurea (SU) herbicides (azimsulfuron, bensulfuron-methyl, and imazosulfuron) and imidazolinone herbicides (imazapyr and imazaquin) (Yong et al., 2002).
Monochoria vaginalis is a monocot weed in the Pontederiaceae family. M. vaginalis is one of the most dominant paddy weeds globally and evolved resistance to ALS inhibitor bensulfuron-methyl in Japan, China, and Korea (Heap, 2022). Although its competitive effect on rice is not as high as Echinochloa crus-gall it is still a weed of equal importance to E. crus-galli (Won et al., 2014). Since the first report of herbicide-resistant M. vaginalis in Korea in 1999 (Kwon et al., 2000), all newly developed herbicides had to contain active ingredients capable of controlling herbicide-resistant M. vaginalis.
4-Hydroxyphenylpyruvate dioxygenase (HPPD) inhibitors are a class of herbicides that inhibit plant growth by blocking 4-hydroxyphenylpyruvate dioxygenase, which is an enzyme in plants that breaks down the tyrosine into molecules. These groups of herbicides were primarily used in rice production in Japan. HPPD inhibitors have been used for corn, soybean, and cereal in Europe and North America since the late 1990s, and have become more important as weeds have shown resistance to glyphosate and other herbicides. However, constant use of the HPPD inhibiting herbicides leads to a selection of the HPPD inhibiting herbicide-resistant weed population. The first HPPD inhibitor resistance case have been detected in Amaranthus tuberculatus in a corn field in 2009 and 14 species of weeds have been reported to be resistant to HPPD inhibiting herbicides worldwide to date (Heap, 2022). HPPD inhibitors have been registered (KCPA, 2007) and used in paddy fields of Korea, but no weed species have been reported so far as resistant to these herbicides. Even so, early detection of resistance risk to this group (HPPD) of herbicides in paddy weed species (S. juncoides and M. vaginalis) is also important as part of herbicide resistance management.
Baseline sensitivity is the beginning point of the average sensitivity of the weed to the specific chemical compound and can ensure herbicide resistance criteria of any herbicide (Espeby et al., 2011). Espeby et al. (2011) and Kanetis et al. (2008) pointed out that the main purpose of the baseline sensitivity study is the determination of natural variation in herbicide or pesticide sensitivity of the population in the target area. Basic and important information such as application timing and/or recommended dose of newly registered herbicides (Paterson et al., 2002) or insecticides (Wise et al., 2008; Wong et al., 2010; Yuan et al., 2006) can be obtained by a baseline sensitivity study. In addition, the baseline sensitivity study can be put into practice to predict the risk of development of herbicide resistance even before new herbicides are registered and this can be one way to establish effective weed management strategies (Paterson et al., 2002; Vidotto et al., 2007). The response of each weed species to a particular herbicide varies between populations (Lim et al., 2021) and the possible herbicide resistance in the species is closely linked with baseline sensitivity and genetic diversity (Blows and Hoffmann, 2005; Moss, 2017). Thus, understanding the baseline sensitivity of certain weeds to specific herbicides is important and baseline sensitivity allows evaluation of the potential risk of developing herbicide resistance in the particular weed. Therefore, this study was conducted to evaluate the baseline sensitivity of S. juncoides and M. vaginalis to HPPD inhibitors, benzobicyclon, and mesotrione to estimate the potential risk of herbicide resistance in rice paddies of Korea.
Materials and Methods
Seed source
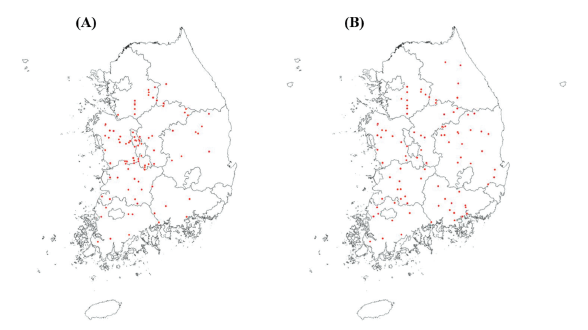
The seeds of S. juncoides and M. vaginalis at maturity were collected from paddy fields in Korea before rice harvesting in October 2020 (Fig. 1). Global positioning system (GPS) information and addresses of sampling sites were recorded using ICE CPS 100c (Supplemental data 1 and 2). To break dormancy, seeds were placed in a 50-mL tube (SPL Life Science Co., Ltd., Seoul, Korea) and kept dipping in the water for one month in the refrigerator at 4℃. The water in the tube was changed to distilled water once a week regularly.
The dose-response assay
The whole plant dose-response experiment was conducted at the experimental glasshouse (E127°35'4.0'' longitude and N36°36'99.5'' latitude) of Chungnam National University located in Daejeon, South Korea in 2021. Three to five seeds of each biotype were sown into a perforated tray (equipped the 105 holes) filled with paddy soil (Seoul Bio Co., Ltd., Seoul, Korea). These trays were placed in a rectangular polystyrene pot (L: 56 cm, W: 35 cm, H: 15 cm) under flooded conditions (3 cm water depth) and incubated with metal halide light to provide a 12-h photoperiod from 6 a.m. to 6 p.m. at 25/30℃ light/dark in the greenhouse. Two HPPD inhibitors including mesotrione and benzobicyclon were applied as water surface application when the weeds were at the 2-3 leaf stage. Mesotrione (40% EC; Tenor cityTM, Syngenta Co., Ltd., Seoul, Korea) was applied at 5, 10, 20, 40, 80, 160 g a.i. ha-1 and benzobicyclon (3.5% EC; NajimaTM, KyungNong Co., Ltd., Seoul, Korea) was applied at 4.4, 8.8, 17.5, 35, 70, 140 g a.i. ha-1 to both S. juncoides and M. vaginalis biotypes. Without herbicide was used as untreated control. The aboveground fresh weight of the treated weeds was measured 14 days after treatment (DAT). The experiment was a Completely Randomized Block design with three replications.
Statistical analysis
Non-linear regression analysis was performed by fitting the fresh weight of samples measured 14 DAT. The log-logistic model estimated the GR50 (the dose requiring 50% fresh weight reduction) values of S. juncoides and M. vaginalis.
The data were expressed as the percentages of untreated control estimates. A non-linear regression dose-response equation (1) (Streibig, 1980) was used in OriginPro 8.1 (OriginLab, 2021) program.
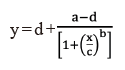
where y is fresh weight; x is the dose rates of herbicides; a is the minimum value; d is the maximum value; c is GR50 (the herbicide dose required for 50% biomass reduction compared with the untreated); b is proportional to the slopes around the dose of GR50.
The baseline sensitivity index (BSI) was calculated by dividing the greatest GR50 value (GR50max) by the smallest GR50 value (GR50min) as follows,
BSI=GR50max/GR50min (2)
One-way ANOVA statistical analysis was performed using OriginPro 8.1 (OriginLab, 2021).
Results and discussion
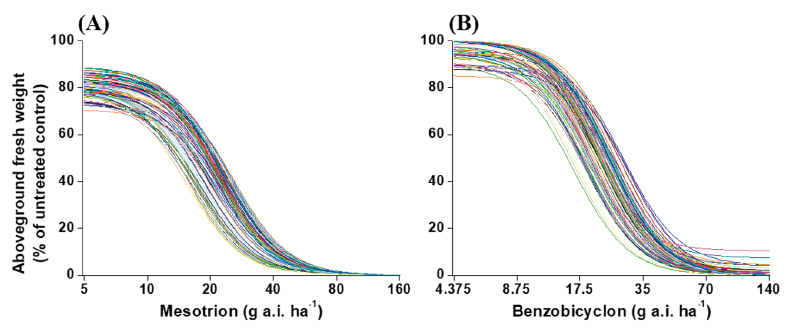
Whole-plant assays and nonlinear regression analysis revealed the dose responses and BSI of S. juncoides and M. vaginalis to benzobicyclon and mesotrione. In S. juncoides, over 90% growth reduction was observed in 105 tested biotypes at the recommended dose of mesotrione (160 g a.i. ha-1) and 104 out of 105 biotypes at the recommended dose of benzobicyclon (140 g a.i. ha-1) (Fig. 2). The GR50 values of S. juncoides ranged from 9.6 to 16.5 g a.i. ha-1 to mesotrione and 13.1 to 26.4 g a.i. ha-1 to benzobicyclon, resulting in the BSI of 1.72 and 2.01 (Table 1).
|
Table 1. Baseline sensitivity indices of Scirpus juncoides populations to mesotrione and benzobicyclon in Korea. 
|
|
*GR50: The rate causing a 50% growth reduction. |
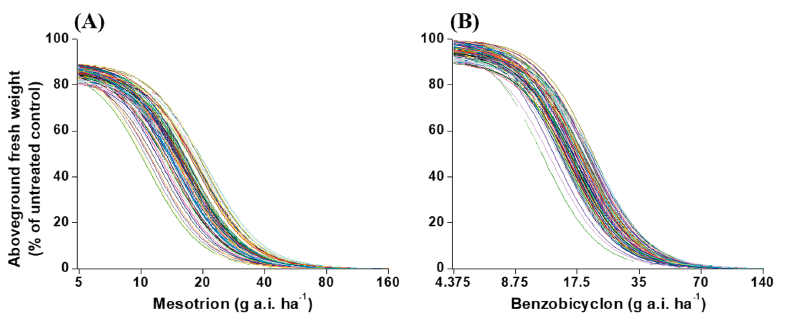
The experimental data showed that all of the 105 (100%) tested M. vaginalis biotypes were significantly controlled with over 90% growth reduction in fresh weight compared to untreated control at the 1x recommended dose of both herbicides (Fig. 3). The value of GR50 in M. vaginalis ranged from 10.9 to 16.3 g a.i. ha-1 to mesotrione and 11.7 to 21.1 g a.i. ha-1 to benzobicyclon. The BSI was 1.48 and 1.78 for mesotrione and benzobicyclon respectively (Table 2).
To compare variations in the sensitivity of weed biotypes to mesotrione and benzobicyclon, the GR50 values of S. juncoides (Fig. 4) and M. vaginalis (Fig. 5) were arranged from the lowest to highest. GR50 values among biotypes of both species to mesotrione and benzobicyclon were not statistically differed in one-way ANOVA Scheffe’s test.
|
Table 2. Baseline sensitivity indices of Monochoria vaginalis populations to mesotrione and benzobicyclon in Korea. 
|
|
*GR50: The rate causing a 50% growth reduction. |
Our study found a less-potential occurrence of the mesotrione and benzobicyclon resistance cases in both weed species. In the case of S. juncoides, minimum and maximum GR50 values were not highly different and BSI for mesotrione and benzobicyclon was 1.72 and 2.01, respectively (Fig. 4). For comparison, the first SU-resistant biotypes of S. juncoides were found in rice fields located in Hokkaido Prefecture, Japan and the lethal dose of the resistant biotype was 40-140 fold for bensulfuron-methyl, 41-79 fold for pyrazosulfuron ethyl, and 75-93 fold higher for imazosulfurone than that of the susceptible biotype (Kohara et al., 1999). Tanaka (2003) reported that imazosulfuron controlled the sensitive S. juncoides biotype above 80% at the dosage of more than 10 g a.i. ha-1 but could not control the resistant biotype at 1,000 g a.i. ha-1. The rates required to inhibit 50% growth of resistant biotype were 271 fold higher than that of susceptible one.
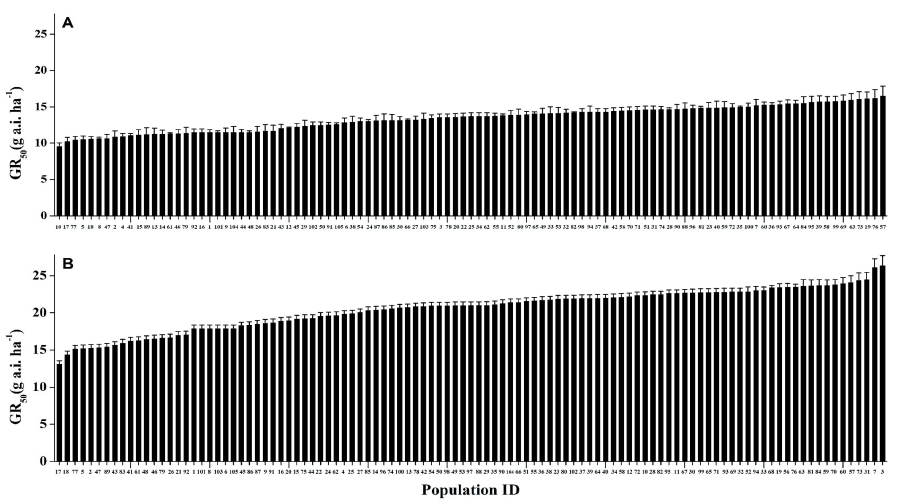
Fig. 4. GR50 values of Scirpus juncoides populations to mesotrione (A) and benzobicyclon (B) in Korea. Vertical bars represent the means of three replications (n=3). P-values (>0.05) were not statistically significant in the one-way ANOVA Scheffe’s test. GR50: The rate causing a 50% growth reduction.
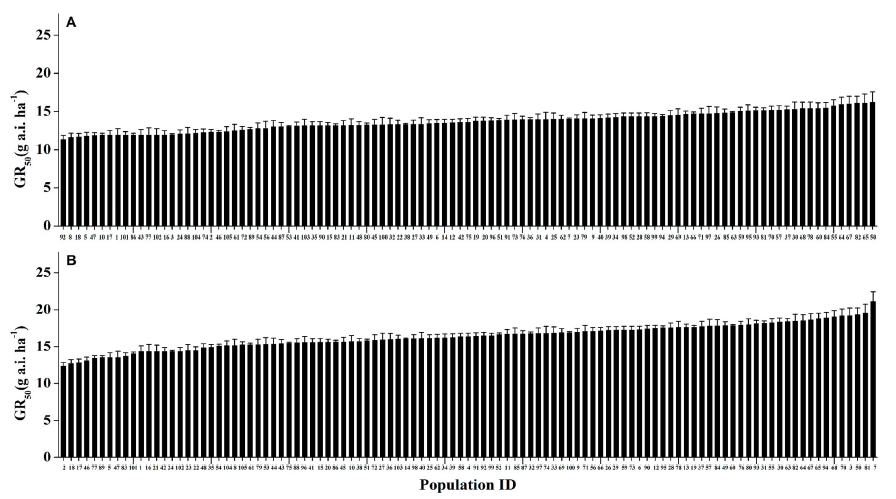
Fig. 5. GR50 values of Monochoria vaginalis populations to mesotrione (A) and benzobicyclon (B) in Korea. Vertical bars represent the means of three replications (n=3). P-values (>0.05) were not statistically significant in the one-way ANOVA Scheffe’s test. GR50: The rate causing a 50% growth reduction.
In our study, no existing resistance cases to mesotrione and benzobicyclon were observed in M. vaginalis biotypes in Korea. BSI was also relatively low, having 1.48 for mesotrione and 1.78 for benzobicyclon (Fig. 5). According to Kurniadie et al. (2021), the percentage of weed growth reduction varies from place to place and is influenced by several factors such as land-use history, farmers’ herbicide application practices, frequency of herbicide application, and crop planting patterns. However, if one of the biotypes is not resistant to a particular herbicide, the difference between GR50max and GR50min values will not be significant. Kurniadie et al. (2021) performed a whole plant resistance test on M. vaginalis to ALS inhibitor, bensulfuron-methyl, and found resistant biotypes with resistance ratios ranging from 12.6 to 48.76. Kuk et al. (2002) reported that GR50 values of resistant M. vaginalis biotype to imazosulfuron were 1,112-3,172 fold higher compared with susceptible biotype in the whole-plant response bioassay. Several researchers have been conducted baseline sensitivity studies on different species to different herbicides. For instance, Papaver rhoeas to florasulam (Paterson et al., 2002), Echinochloa crus-gall to azimsulfuron, bensulfuron-methyl, cyhalofop-butyl, molinate, propa,nil and quinclorac (Vidotto et al., 2007), Alisma plantago-aquatica, Cyperus difformis, and Schoenoplectus mucronatus to penoxsulam (Loddo et al., 2018), Lolium rigidum and Bromus diandrus to glyphosate (Barroso et al., 2010) was conducted globally. In Korea, Lim et al. (2021) reported the baseline sensitivity index of Echinochloa crus-gall and E. oryzicola to florpyrauxifen-benzyl, a new synthetic auxin herbicide. The baseline sensitivities of Echinochloa crus-gall to the very-long-chain fatty acid synthase (VLCFAs) inhibitors (mefenacet, pretilachlor, fentrazamide, and cafenstrole), PPO inhibitor (oxadiargyl), and herbicide with unknown mode of action (oxaziclomefone) were also conducted (Lim, 2013).
Based on the results obtained from our experiment, we predicted that there is a relatively low risk of the development of mesotrione and benzobicyclon resistant S. juncoides and M. vaginalis biotypes in Korea. The reason why resistance to the HPPD inhibiting herbicides has not yet evolved despite their quite long-term use in Korea might be the use of herbicides with a different mode of action in rice fields. However, we emphasize that farmers do not use sublethal doses or continuous use of mesotrione and benzobicyclon in the rice fields since repeated usage or sublethal doses of certain herbicide applications may lead to herbicide resistance (Ashworth et al., 2016). As an example, Palmer amaranth evolved resistance to dicamba as a result of lower dose application (Crespo et al., 2016) and the continuous use of 2,4-D for more than 12 years, resulting in a 2,4-D resistant Amaranthus tuberculatus population (Bernards et al., 2012). Interestingly, in the USA, HPPD inhibitor-resistant Palmer amaranth populations were observed in a cornfield, which had a history of continuous application and even with no previous history of use of HPPD herbicides (Sandell et al., 2012). This means, the population was not evolved resistance to HPPD inhibiting herbicides, but earlier used herbicides with different modes of action and exhibited cross-resistance to HPPD inhibiting herbicides. Lu et al. (2020) reported the cross-resistant wild radish population to the HPPD inhibiting herbicides mesotrione, tembotrione, and isoxaflutole which had shown 4 to 6.5-fold resistance compared to the susceptible population in the dose-response experiments.
Conclusion
In conclusion, the baseline sensitivity index for S. juncoides and M. vaginalis was relatively low (1.48-2.01) to HPPD inhibiting herbicide, mesotrione, and banzobicyclon. No shift in mesotrione and benzobicyclon sensitivity was observed suggesting that these HPPD inhibitors can still be used very effectively to control S. juncoides and M. vaginalis populations in rice fields in South Korea. However, HPPD inhibitor-resistant paddy weeds may occur considering that HPPD inhibiting herbicides have been used in cropping systems for more than 15 years (KCPA, 2007) in Korea. Therefore, constant monitoring is necessary to recognize the evolution of herbicide-resistant weed biotypes.
Acknowledgments
This work was carried out with the support of Syngenta Korea and Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry (IPET) through Agricultural Machinery/Equipment Localization Technology Development Program, funded by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry (IPET) (321056-05).
Authors Information
In Kon Park, Syngenta Korea, Postdoctoral Researcher
Umurzokov Mirjalol, https://orcid.org/0000-0001-8148-8041
Aung Bo Bo, https://orcid.org/0000-0001-7579-3429
Hoyong Shin, Gyeongsang National University, Master student
Kwang Min Cho, https://orcid.org/0000-0003-0537-2164
Kee Woong Park, https://orcid.org/0000-0003-0053-9543
Jeung Joo Lee, Gyeongsang National University, Professor