Introduction
Weeds are a serious problem in agricultural crop production, causing higher yield losses. Continuous use of synthetic herbicides in weed control exacerbates long-term ecological problems such as environmental pollution, harmful herbicide residue accumulation in soil and water resources, mammalian toxicity, and the evolution of herbicide resistance in prevalent weed species (Yasuor et al., 2008). During the last fifty years, heavy reliance on chemical herbicides has increased crop productivity with prominent ecological and economic impacts (Cordeau et al., 2016). After numerous environmental problems due to the detrimental impacts of chemical herbicides, researchers investigated alternative biological control systems (Dhanasekaran et al., 2012).
Bioherbicides are compounds that are derived from microorganisms such as fungi, bacteria, viruses, and also some plant species. Secondary metabolites produced by these species cause plant phytotoxic activities such as necrosis, chlorosis, deformation, and stunting (Daniel et al., 2008; Umurzokov et al., 2021). These features are prerequisites to use as bioherbicides for weed control (Cai and Gu, 2016). Furthermore, more effective compounds can be found by deeper exploring the quantitative structure of active metabolites generated by microorganisms (Dayan et al., 2008). Therefore, since the 1990s, scientists have focused on bacterial herbicides to control weeds (Ji et al., 2006). However, weak herbicidal activity is the main constraint towards the development and large-scale use of bioherbicides (Basilio et al., 2003). Recent findings revealed that phytotoxic metabolites vary in their degree of target-specificity, though weed species may also differ in susceptibility to a particular toxin (Todero et al., 2018).
Soil microorganisms can produce valuable secondary metabolites including bioactive herbicidal compounds that might be used to create as a good candidate for biological weed control (Christy et al., 1993). Among them, Streptomyces are a major group of inhabitant phytopathogenic bacteria with herbicidal or insecticidal activity living in soil (Kim et al., 2022; Nakajimam et al., 1991). For example, Bilanaphos has been commercialized as a herbicide in 1983, which constituted the extracts of Streptomyces hygroscopicus SF1293 (Xu et al., 2009). Moreover, glufosinate-ammonium formulated with the metabolites produced by Streptomyces hygroscopicus was successfully commercialized in 1984, and currently, it is at the top of 10 bioherbicides that have been used all around the world (Ha et al., 2017). Blasticidin S constitutes the extracts of Streptomyces griseohromogenes, herbicidin A and B consist of S. saganonensis. and S. scopuliridis; and nigericin, hydantocidin and geldanamycin were developed as bioherbicides from the active compounds of S. hygroscopicus (Nakajimam et al., 1991).
However, the phytotoxic activity of Streptomyces as a target-specific bioherbicide was not thoroughly studied in Korea. The objectives of the research were to isolate and to identify Streptomyces from soil and to determine the herbicidal activity of Streptomyces strain W-200 against weeds. In addition, the herbicidal activity of Streptomyces strain W-200 was assessed with various optimal culture conditions and their mode of action following the foliage treatment and compared to common chemical herbicides to evaluate its potential as a new bioherbicide.
Materials and Methods
Soil sample collection
Soil samples were collected from the rhizosphere of plants at a depth of 10-20 cm in different forests, and non-agricultural lands in Gangwon-do, Gyeonggi-do, Chungcheonbuk-do, Chuncheongnam-do, Gyeongsangbuk-do, Jeollabuk-do, and Gyeongsangnam-do in April 2018 and kept at 4℃ in the laboratory. Each sample was air-dried at room temperature and passed through a 2 mm-mesh sieve (Hamedi et al., 2014).
Isolation and culture preparation of Streptomyces
One gram of each sample was suspended in 9 mL of distilled water in a sterile test tube and vortexed. Then, the soil solutions were subjected to serial dilutions up to 10-3. Then, 100 μL aliquot of the dilutions was spread out on humic acid-vitamin (HV) agar (20 g bacteriological agar, 0.5 g MgSO4, 5 g Na2HPO4, 0.2 g CaCO3, 10 g humic acid, 20 g KCL, 5 g cycloheximide, and 1 L distilled water). The culture plates were incubated at 25℃ in the dark for 14 days until the sporulation of the bacterial colonies. Representative colonies of Streptomyces were picked and streaked on fresh Bennet’s agar plates (10 g glucose, 1 g yeast extract, 2 g peptone, 1 g beef extract, 20 g bacteriological agar, and 1 L distilled water) and incubated at 25℃ for 7 days (Valanarasu et al., 2009). Pure colonies of the Streptomyces isolates from Bennet’s agar were cultured in fresh M3 liquid medium (20 g soytone, 20 g glucose, 40 g soluble starch, 6 g CaCO3, and 1 L distilled water) and incubated in a rotary shaker (160 rpm) at 27℃ for 7 days. The biomass of the bacteria was harvested by centrifuging the fermentation broth for 10 min (7,871 relative centrifugal force [RCF]) at 4℃.
Screening for bio-herbicidal assay
Streptomyces isolates were subjected to Digitaria ciliaris to verify the bio-herbicidal activity under the greenhouse condition (30/25±5℃, light/dark=14/10 h). D. ciliaris is an important weed in upland crops. If this weed is not controlled, it may cause high yield losses. The sowing of D. ciliaris was carried out with 20-25 seeds per cup in horticultural soil under the greenhouse condition for 7-10 days and 5 mL culture of Streptomyces supernatant (×1) was sprayed on the foliage D. ciliaris. In all tests, 0.1% Tween20 was added to disrupt the water surface tension. Herbicidal activity was ascertained visually in comparison with untreated control plants at 7 days after treatment (DAT). The evaluation of physiological and morphological symptoms of mortality was detected based on the mortality percentage of the Brazilian Society of Weed Science (SBCPD) (0%=no injury, 20-40%=slight injury or effect or reduction of growth with rapid recovery, 40-60%=moderate injury or reduction of growth with slow recovery or definitive, 60-80%=severe injury or reduction of non-recoverable growth and/or reduction of the stand, 80-100%=complete destruction or only a few live plants).
16S rRNA gene sequence analysis
The Streptomyces strain which had the highest herbicidal activity was characterized by 16S rRNA gene sequencing. The bacterial 16S rRNA was amplified using universal primers 27F (5´-AGAGRR TGARCCTGGCTCAG-3´) and 1492R (5´-GGR TACCTTGRRACGACTT-3´). Genomic DNA was obtained from a single colony of bacterial cells disrupted by heating at 100℃ for 10 min. For the spore-forming isolate, the bacterial cell was disrupted in a microwave at 650 W for 30 s in a buffer. The suspensions were centrifuged at 13,000 rpm for 10 min. After centrifugation, 2 μL of the supernatants were used for polymerase chain reaction (PCR). The PCR products were purified and sequenced at SolGent Inc. (Daejeon, Korea). The resulting sequences were analyzed with the BLASTn program of NCBI (http://www.ncbi.nlm.nih.gov). The phylogenetic tree was constructed by the Neighbor-joining method using the program MEGA 6.0 (Saitou and Nei, 1987).
Herbicidal spectrum experiment for weeds contro
Seeds of different weeds species (Echinochloa crusgalli, D. ciliaris, Setaria viridis, Lolium multiflorum, Amaranthus retroflexus, Solanum nigurm, Abutilon theophrasti, Aeschynomene indica) (20 seeds per pot with 3 replications) were planted 1-2 cm deep in pots containing commercial horticultural soil under the greenhouse condition.
The concentrations of culture filtrate of Streptomyces strain W-200 were diluted to 25, 50, 75, and 100% for soil and foliar applications. For both applications, each culture filtrate with a volume of 5 mL was sprayed for each replication. Distilled water was used as the control. The experiment was conducted in a completely randomized design with three replications. The effect of phytotoxic activity was evaluated at 14 DAT for soil application and 7 DAT for foliar application. Germination percentage of the seeds was assessed for each replication by the equation:
G = ×100 (1)
×100 (1)
Establishing the culture conditions
To test the herbicidal activity of Streptomyces strain W-200, the bacterial isolate was inoculated into a 500 mL Erlenmeyer flask containing 200 mL M3 liquid medium. After inoculation, the flasks containing bacterial strain were incubated at different temperature ranging from 20, 25, 30, and 35℃ by shaking incubation at 160 rpm, various agitation speeds ranging from 50, 100, 150, and 200 rpm, and adjusted initial pH values of (3, 5.5, 7, 8.5, and 10) by keeping at 27℃ for 7 days on a shaker, and then the supernatants of culture filtrate were tested on the growth of D. ciliaris. A visual assessment was conducted at 7 DAT to evaluate physiological and morphological symptoms of mortality.
Herbicidal activity of W-200 under light and dark conditions
The seeds of D. ciliaris were germinated in a greenhouse condition (30/25±5℃, light/dark=14/10 h) for 2 weeks. The culture filtrate of Streptomyces strain W-200 was prepared at ×0.25, ×0.50, ×0.75, and ×1 application rates containing 0.1% Tween 20 for post-emergence application. Then, 5 mL of each rate was sprayed directly onto the foliage and stem of D. ciliaris. The treated plants were kept in 24 h continuous light and dark growth chambers at 25℃. The bioherbicidal effects of Streptomcyes in each concentration were evaluated visually 7 DAT to determine the morphological and physiological damages assessed by the mean percentage of three mortality score replicates.
Photosynthesis inhibition
Seeds of D. ciliaris were grown in pots (6×9 cm) for two weeks in a greenhouse condition (30/25±5℃, light/dark=14/10 h). The culture filtrate of Streptomyces strain W-200 at ×1 rate, paraquat (1,155 g a.i. ha-1), and glufosinate-ammonium (900 g a.i. ha-1) were applied to the plant at 2-3 leaf stage. After application, the treated plants were incubated in the growth chamber (30/25±5℃, light/dark=12/12 h). The inhibition rate of photosynthesis in the foliage of D. ciliaris was measured by a chlorophyll fluorescence meter (Handy PEA, Hansatech Instruments, King’s Lynn, UK) 24 h after application. All treatments had three replications in each trial.
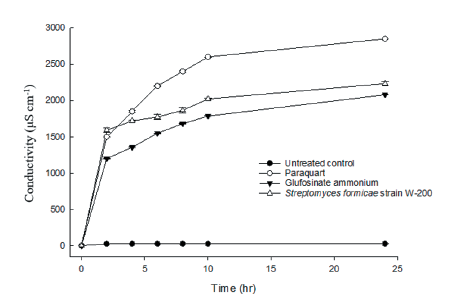
Electrolyte leakage assay
The electrolyte leakage assays were performed on one-week-old Cucumis sativus seedlings. A total of 30 leaf discs (about 5 mm diameter) were placed into a Petri dish containing 1 mM of MES buffer solution, then, each 5 mL culture filtrate of Streptomyces, paraquat (1,155 g a.i. ha-1), and glufosinate-ammonium (900 g a.i. ha-1) were added into the Petri dish. An untreated control was prepared in the same way as the treated materials except it received 5 mL of water. Following the treatment, the Petri dishes were placed in a growth chamber under 25℃ day-1 for incubation. Electrolyte leakage was measured at 0, 2, 4, 6, 8, 10, and 24 hours after treatment using an electronic conductivity meter (TOA-DKKCm-21P, DKK TOA, Tokyo, Japan).
Statistical analysis
Statistical analysis was performed using Sigma Plot 14.0 software, considering a significance level of 95%. For the bio-herbicidal assay on C. sativus, each treatment was composed of 15 plants with three replicates of five plants. For the bio-assay on the growth of D. ciliaris, each treatment consisted of 20-25 plants in each replicate of three replications.
Results
Screening Streptomyces isolates with bioherbicidal activity
In the screening, 16 out of 332 bacterial isolates (4.8%) showed considerable phytotoxic activity on the growth of D. ciliaris. In the potential 16 bacterial isolates, there were 4 isolates in Gungwon-do, 10 isolates in Gyeongsangbuk-do and Chungcheongnam-do, and the remaining 2 bacterial isolates from Gyeongsangnam-do.
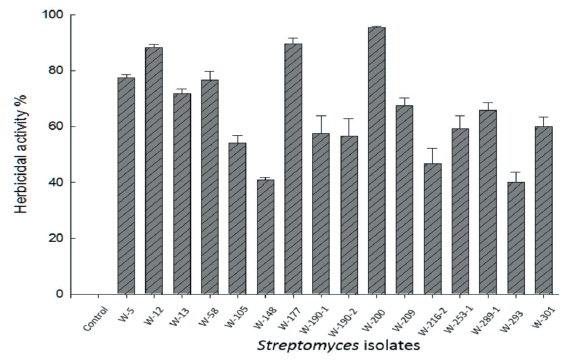
Among the selected 16 isolates, the three isolates namely W-12, W-177, and W-200 exhibited considerable activity against the growth of D. ciliaris (Fig. 1). As estimated, the plant growth of D. ciliaris was reduced by 88.3%, and 89.67% after the application of isolate W-12 and W-177, respectively, whereas isolate W-200 reduced to the plant growth of D. ciliaris by 95.50% at 7 DAT.
The observation of isolates W-12 and W-177 revealed herbicidal activity but did not completely inhibit the plant growth. The host range test conducted on these three isolates showed that isolate W-200 reduced the plant growth with severe injury such as yellowing and blight on the foliage of D. ciliaris, which eventually led to the plant death (Fig. 2). Therefore, taking into account substantial herbicidal effect morphology of the target weeds, isolate W-200 was selected for molecular identification and further investigation to determine as a potential bio-herbicide.
16S rRNA gene sequencing of Streptomyces strain W-200
In this study, the isolated Streptomyces strain W-200 was identified by 16S rRNA gene sequencing with PCR technique using universal primers. Phylogenetic analysis of Streptomyces strain W-200 was constructed based on the neighbor-joining using its 16S rRNA sequence with that of 15 Streptomyces spp. The data of nucleotide sequence has been set down at GenBank under the accession number NR146048.1. Based on the BLAST results, the isolated Streptomyces strain W-200 was 99% similar to Streptomyces formicae (1H-GS9) as shown in Fig. 3.
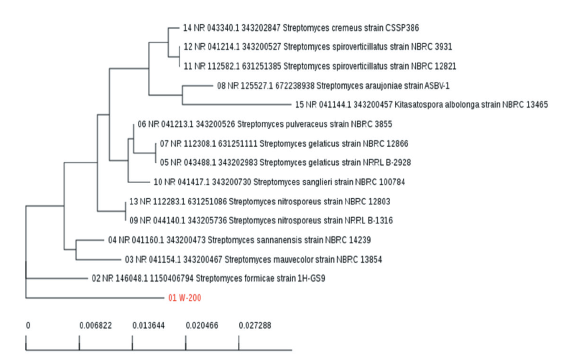
Fig. 3. Neighbor-joining phylogenetic tree of Streptomyces formicae strain W-200 that produced bioherbicidal metabolite. Phylogenetic analysis was accomplished by MEGA 6.0 based on 16S rRNA sequences of W-200 and 15 species of related Streptomyces. Bootstrap values, expressed as a percentage of 1,000 replications, are giving at branching points. Bar 0.006 substitutions per nucleotide position.
Herbicidal activity of Streptomyces strain W-200 in soil and foliar applications
In the case of soil application, seed germination of D. ciliaris was sensitive in all concentrations of Streptomyces strain W-200 (×0.25: 30±2.13, ×0.5: 10±1.53, ×0.75: 0±0.50, ×1: 0±0.00) compared with untreated control (95±1.25). In general, Streptomyces strain W-200 revealed more herbicidal activity and growth inhibitory effect in grass weed species than in broadleaf weed species. The greenhouse experiment showed that the culture filtrate of Streptomyces strain W-200 had the most effective herbicidal activity on the foliage of D. ciliaris at all concentrations (×0.25: 36.67±5.77, ×0.5: 45±15.00, ×0.75: 75±5.00, ×1: 90±5.00) compared with untreated control at 7 DAT (Table 1).
|
Table 1. Inhibition of seed germination and level of injury as affected by Streptomyces strain W-200. 
|
Effect of temperature, agitation, and pH on the growth of Streptomyces
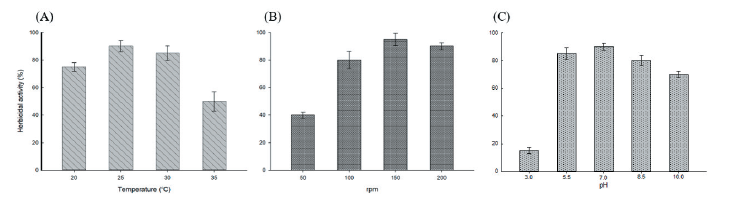
Streptomyces strain W-200 was cultivated at the above described different temperatures (20, 25, 30, and 35℃), agitation speeds (50, 100, 150, and 200 rpm), and pHs (3.0, 5.5, 7.0, 8.5, and 10.0). The results demonstrated that the Streptomyces strain showed high herbicidal activity at temperatures between 25-30℃ while activity decreased at higher temperatures, agitation speed between 150-200 rpm, and pH with a value between 5.5-7 while herbicidal activity was showed decreasing at higher pH values (Fig. 4).
Herbicidal activity of W-200 under light and dark conditions
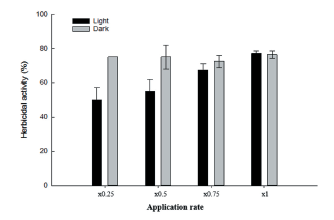
In this experiment, herbicidal symptoms on the growth of D. ciliaris were compared in light and dark conditions in the growth chamber after the application of Streptomyces strain W-200. Generally, the damage of leaves increased with increasing the concentrations of culture filtrate in both conditions (Fig. 5). However, the herbicidal activity of Streptomyces strain W-200 in light condition slightly increased than that of dark condition. As the level of injury, culture broth of Streptomyces strain W-200 controlled over 70% of the growth of D. ciliaris in both conditions.
Influence on chlorophyll content
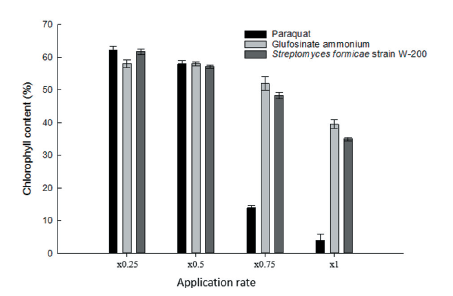
Photosynthesis inhibitor herbicide suppresses light-dependent reactions and electron transport systems in chloroplasts, which leads to the accumulation of active oxygen, followed by oxidation of cell membranes and subsequent necrosis. The chlorophyll content of D. ciliaris applied with Streptomyces strain W-200, paraquat, and glufosinate-ammonium was evaluated with a chlorophyll fluorescence meter. It was observed that the chlorophyll content decreased with increasing concentrations. In the case of paraquat, the chlorophyll content decreased sharply. But, in the cases of Streptomyces strain W-200 and glufosinate-ammonium, it was slight variation compared with paraquat. The D. ciliaris treated with paraquat (×1 rate) showed that chlorophyll content decreased 96% compared with untreated control. On the other hand, culture filtrate of Streptomyces strain W-200 and glufosinate-ammonium at ×1 application rate were observed to have 65% and 61% reduction in chlorophyll content, respectively, compared with untreated control (Fig. 6). On the basis of the result, it was assumed that the culture filtrate of Streptomyces strain W-200 inhibit photosynthesis.
Electrolyte leakage
To know the disruption of the cell membrane in plant, an electrolyte leakage assay was performed after treatment of Streptomyces strain W-200, paraquat, and glufosinate-ammonium. In this experiment, electrolyte leakage occurred rapidly from 2 to 24 h after paraquat treatment whereas it was observed slower after treatment with culture filtrate of Streptomyces strain W-200 and glufosinate-ammonium, but the rapid increase was found from 4 to 10 h (Fig. 7). Accordingly, the amount of electrolyte leakage induced by Streptomyces strain W-200 was not as rapid as that induced by paraquat. However, it was slightly higher than the glufosinate-ammonium, but the difference did not reach the significant level at 24 h. Twenty-four hours after treatment of three herbicidal products, paraquat was identified as the largest amount of electrolyte leakage followed by culture filtrate of Streptomyces strain W-200 and glufosinate-ammonium.
Discussion
Biological weeds control using soil microorganisms is an essential alternative control method to a chemical treatment to reduce weed capacity, density, and harmful effects. The present study indicated that different Streptomyces strains can be found in soil samples. The isolated Streptomyces strains were evaluated on phytotoxic activity on D. ciliaris aimed the discovery of bioherbicide in the screening. Sixteen isolated strains (4.8%) out of 332 isolates showed some phytotoxic activity on the seedling growth of D. ciliaris. In this assay, only three isolates namely W-12, W-177, and W-200 exhibited considerable activity against to the growth of D. ciliaris. However, the phytotoxic effects of bacterial strains W-12 and W-177 may not necessarily cause the same effect on D. ciliaris as strain W-200 because each bacterial strain has a specific function through the toxification of weed cells. Therefore, isolated bacterial strain W-200 was conducted in the substantial herbicidal experiments.
Dhanasekaran et al. (2012) mentioned that PCR-based molecular methods namely 16S rRNA, randomly amplified polymorphic DNA (RAPD), and short tandem repeat (STR) analyses can be utilized at taxonomic levels. Among the molecular analysis, the 16S rRNA gene sequence is a more suitable and time-saving approach to identifying Streptomyces (Park et al., 2006). Nevertheless, sequencing of genes encoding 16S rRNA is the most promising technique for the phylogenetic classification of bacteria (Bo et al., 2019). Phylogenetic analysis of 16S rRNA gene sequencing determined that isolate W-200 has 99% similarity to Streptomyces formicae (1H-GS9).
The inhibitory effects of microbial isolates on the target weeds are due to the presence of phytotoxic compounds (Bo et al., 2019). Such activity was more visible after the application of Streptomyces strain W-200 in this experiment, which caused damage such as yellowing, following by wilting and complete collapse of D. ciliaris. Susceptibility of weed species varied according to bio-herbicidal activity, on the other hand, herbicidal metabolites also differ in functioning on target-specificity (Hoagland et al., 2016). Therefore, the herbicidal effect of Streptomyces strain W-200 had substantial inhibition activity on the growth of weeds and caused visual injury symptoms, demonstrating high efficiency at foliar application.
Furthermore, Kokare et al. (2007) demonstrated that 28℃ is the optimal temperature, while 25℃ was the optimum temperature for maximum production of herbicidal metabolites of Streptomyces strain W-200 in this study. In the culture speed study for herbicidal activity on the growth of D. ciliaris, the cultivation at 150 rpm speed was optimum. In general, when the higher culture speeds, it acts as a stress on the microorganisms strain and form excessive foam in culture filtrate. Alternately, when the lower culture speeds are under 150 rpm, the oxygen cannot penetrate the culture filtrate of Streptomyces. In the present study, the initial pH value was found suitable for maximum production of metabolites near the natural (pH 7). Therefore, this study confirmed this fact as soybean meal was used in the present study to influence the production of herbicidal metabolites, the pH values affect the cellular metabolism and biosynthesis of secondary metabolites in Streptomyces spp. (Ahmed et al., 2001).
In weed control, chlorophyll loss is an indicator of herbicidal activity for active herbicidal metabolites (Kim et al., 2020; Umurzokov et al., 2022). In the chlorophyll assay, Streptomyces strain W-200 and glufosinate-ammonium were not highly different in all concentrations. The amount of chlorophyll loss from paraquat was higher than Streptomyces strain W-200 and glufosinate-ammonium in all tested concentrations of this study. The electrolyte leakage induced by Streptomyces strain W-200 was between that induced by paraquat and glufosinate-ammonium. In this leakage assay, Streptomyces strain W-200 increased gradually with time until 24 h after treatment. Therefore, the results indicated that Streptomyces strain W-200 destroyed the plant cell membrane over time following the treatment.
Acknowledgments
This work was supported by ‘Cooperative Research Program for Agricultural Science & Technology Development’ (Project No. PJ0161282022), Rural Development Administration, Republic of Korea.
Authors Information
Kwang Min Cho, https://orcid.org/0000-0003-0537-2164
Seung Cheol Shin, Chungnam National University, Ph.D. student
Aung Bo Bo, https://orcid.org/0000-0001-7579-3429
Umurzokov Mirjalol, https://orcid.org/0000-0001-8148-8041
WeiQiang Jia, Beijing Fengdo High-Tech Seed Industry, Researcher
Kee Woong Park, https://orcid.org/0000-0003-0053-9543
Jung Sup Choi, Korea Research Institute of Chemical Technology, Postdoctoral researcher
Young Sook Kim, Korea Research Institute of Chemical Technology, Postdoctoral researcher