Introduction
Giant ragweed (Ambrosia trifida) is a broadleaved herbaceous erect summer annual belonging to the Asteraceae family (Abul-Fatih and Bazzaz, 1979). This plant grows to a height beyond 3 m though it is an herb. Early and prolonged emergence characters of giant ragweed cause immense and long-term competitiveness for nutrients, moisture, and light (Abul-Fatih and Bazzaz, 1979; Gibson et al., 2005). Through abating biological diversity, this species suppresses other plants in the site that they established. Compared to other annual species, A. trifida is among the first to emerge in early spring (Abul-Fatih and Bazzaz, 1979). Moreover, the species causes a hay fever to nearby human populations during flowering stage. Giant ragweed is one of the first emerging annual weed along roads, rivers, and near agricultural areas in Korea (KANC, 1999; ME, 2006). In Korea, it was initially detected neighboring the DMZ, which is positioned on the interior locality of the Korean Peninsula. The distribution scope of the plant is now broadened its range from this central region of Korea. Plant biology and geographical distribution of annual plant species are influenced by environmental factors (Bykova et al., 2012). Therefore, seed germination niche that includes temperature, light, and soil depth are need to be studied to control its invasive in established area (Baskin and Baskin, 2014). The germination niches are responsible for transition of species from seed to seedling (Grubb, 1977). The temperature controls rate of germination by affecting the seed dormancy and the rate of the enzymatic activities (Bewley et al., 2013). The response of the weed species to temperature at the germination stage gives its distribution ability in different regions (Chauhan and Johnson, 2010). Germination and dormancy of some seeds are controlled by the quality and quantity of light (Crisraudo et al., 2007; Bewley et al., 2013). Giant ragweed seeds are relatively large and store a sufficient amount of food reserves which increase seed germination under different environmental factors and promote emergence in greater depths of soil (Abul-Fatih and Bazzaz, 1979; Harrison et al., 2007). Moreover, successful propagation of the species is related with seed dormancy. Due to the hard seed coat, the plant seeds possess a physiological dormancy at maturity stage (Baskin and Baskin, 2001).
Materials and methods
Fully matured seeds of A. artemisiifolia,S. angulatus and A. trifida were collected from plants in Cheongju and Chungcheong provinces, from October to November 2018. Freshly harvested seeds of A. trifida were cleaned and stored in paper bags at room temperature (25℃). To break the seed dormancy, the seeds of A. artemisiifolia and A. trifida were wrapped with double layers of moist paper towel, and stored in a fridge at 4℃ for 3 months before starting the experiments. Seeds of giant ragweed were incubated in a 60×15 mm Petri dish (SPL Life Sciences Co., Ltd., Pocheon, Korea) lined with filter papers. Criterion for germination was radical protrusion (except in the seed burial experiment). The two sheets of filter papers were moistened with 5 ml of water in petri dishes. All experiments were replicated three times.
Changes in seed germination after harvest
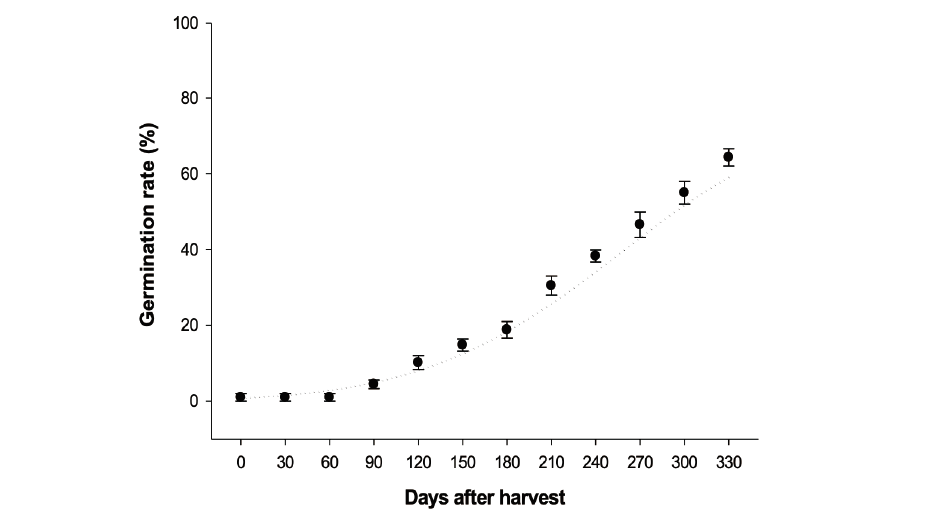
For use in the experiment, seeds of giant ragweed were stored dry at 25℃ during the study period. The seed germination exam started right after harvest (on November 2018) and was carried out for 330 days with 30 days interval.
Effect of photoperiod on seed germination
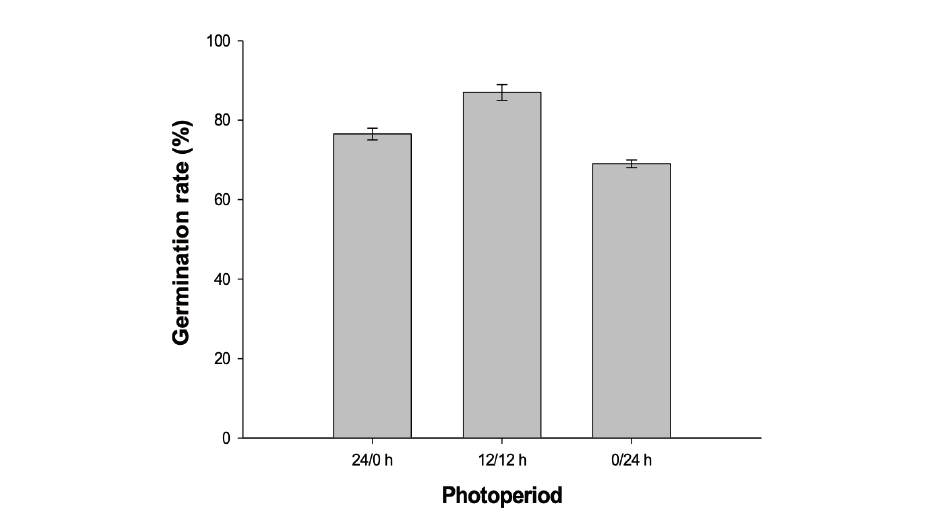
The experiment was conducted under different length of light and dark conditions, establishing either complete light (24/0 h) and complete dark (0/24 h), or alternating light and dark conditions (12/12 h). 20 seeds of A. trifida were spread on wet filter paper in Petri dishes (60×15 mm). To provide full darkness, aluminum foil roll was used as a light-preventer to cover Petri dishes. The temperature in incubator was maintained at 25/15℃ during the experiment.
Effect of temperature seed germination
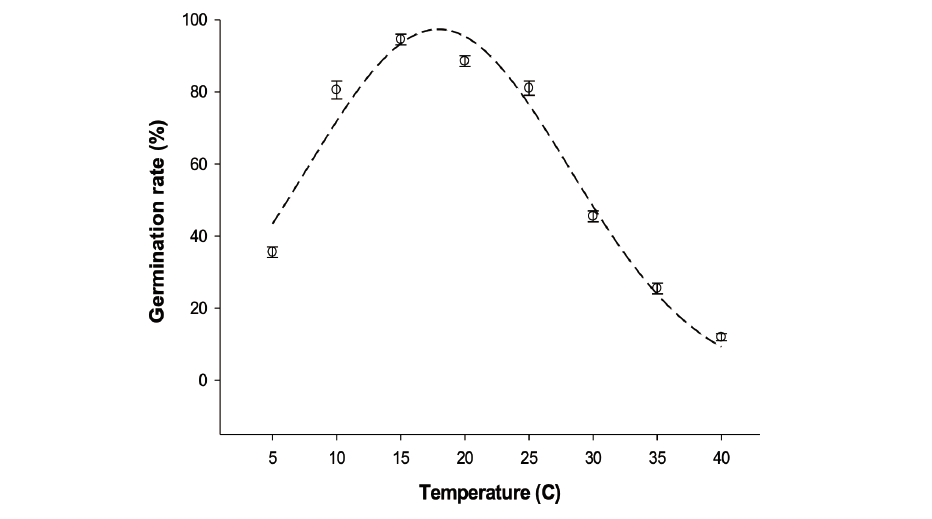
Dishes containing 20 seeds were exposed to eight constant temperatures (5, 10 15, 20, 25, 30, 35, and 40℃) for three weeks. All the subjected seeds in the temperatures were provided equal 12/12 h alternating light/dark conditions. The selected temperature range corresponds to the temperatures that the giant ragweed might experience in Korea.
Effect of seed burial depth on seedling emergence
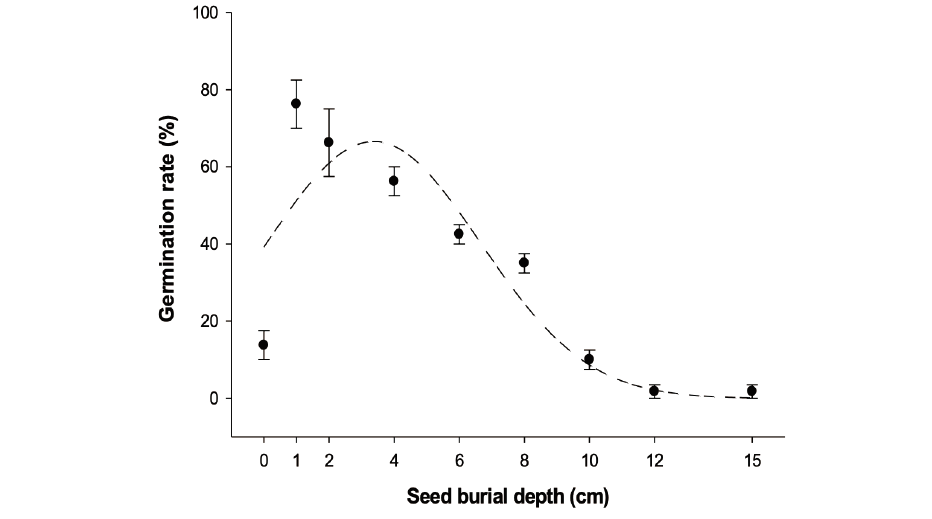
This experiment was conducted under greenhouse condition from 20 April to 13 May 2019. Fifty giant ragweed seeds were placed on the soil surface in pots (25 cm in diameter and 20 cm in height). The seeds in pots were inundated with soil to respective depths of 0, 1, 2, 4, 6, 8, 10, 12, and 15 cm. The soil used in the seed burial experiment was a mixture of organic material and sand (70% sand and 30% organic material, volume ratio). The average temperature in the greenhouse during the study was 21-25℃. The emerged seedling number was counted daily based on cotyledon visibility on soil surface for 21 days. The position of each pot was rotated every week to provide evenness in sunlight availability. The pots were watered every second day to maintain adequate soil moisture. At the end of the trial, the seeds buried deeper than 6 cm retrieved to verify their viability by a pressure test with tweezers (Taylor et al., 2004).
Data analysis
A three-parameter Gaussian model was used to describe the final germination data of changes in seed germination after harvest, temperature and seed burial experiments. The model provides a “Bell curved” figure that fits to downward and upward trends of seed germination in the experiments.
Results and Discussion
Changes in seed germination after harvest
Dry storage of the Ambrosia trifida seeds resulted in a moderate release of seed dormancy, starting from 90 days after harvest (5%) at 25℃. Then there was a steady increase with the average of 6.7% in each trial during the experiment. The maximum seed germination of A. trifida (>65%) was observed by the end of the period at 330 days after harvest. A. trifida seeds required a total of 9 months to achieve 50% germination (Fig. 1). Giant ragweed seeds on the parent plant are dormant at maturity. The embryos of giant ragweed after ripen was slow in dry storage. As Ballard mentioned in 1996, the plant seeds require a cold stratification to break the dormancy under cool and moist soil. Since the breaking the dormancy and germination are continuous physiological processes, it would be difficult to determine a point in time when dormancy ends and germination begins.
Photoperiod
The light had no significant effect on seed germination of the giant ragweed (Fig. 2). The seeds exhibited >60% in either complete light or dark or in alternating light and dark conditions, suggesting that giant ragweed seeds are not strictly photoblastic. The most promoted germination of A. trifida was >86% at alternating light and dark conditions. The results indicating that without a light exposure it can germinate under both shade of the other plants and soil. The light experiment results conformed invasiveness of common ragweed. Exposure to the light stimulates germination in many weed species, but there are species in which light has no effect or even inhibits germination (Baskin and Baskin, 2004).
Temperature
The seeds of A. trifida were able to germinate under all temperatures had been used in the study (Fig. 3). Seed germination rate was increased with increasing temperature until 15℃, and then started to decline. A. trifida seeds germinate well at low temperatures between 5 to 15℃ (35 to 94%) compare to high temperatures from 30 to 40℃ (45 to 12%). Optimum temperature for germination of the plant seeds was between 15 and 25℃ (>85%). The ability of giant ragweed seeds to germinate at low temperatures was not unexpected, since it is one of the first summer annual weeds to emerge under low soil temperature conditions early in spring (Abul-Fatih and Bazzaz, 1979). Although seeds were able to germinate over a wide range of temperatures, seed survival and reproduction are not guaranteed, as a seedling and subsequent growth stages are highly sensitive to adverse environmental conditions (Moles et al., 2005a).
Seed burial
The seedling emergence of A. trifida was significantly influenced by seed burial depth (Fig. 4). The seedling emergence of giant ragweed was greatest (76%) at 1cm layer of the soil and this was slightly greater (66%) than the seeds placed at 2 cm layer. Seedling emergence increased at shallow burial depths of 1 and 2 cm but declined progressively as depth increased. Because of poor soil and seed contact and water imbibition, which resulted in low water uptake caused lower seedling emergence of A. trifida on the soil surface than the emergence of the seeds buried at 1 and 2 cm depths. The emergence of giant ragweed decreased sharply from 4 to 10 cm, resulting in no emergence at the depths of 12 and 15 cm. Moles et al. (2005b) reported that large-seeded species are able to tolerate better to many of the environmental stresses encountered during seedling establishment. Low or no emergence of deeply buried seeds could be due to fatal or no germination. Decreased emergence at increased planting depth has been reported in several weed species, including Conyza Canadensis L. (Nandula et al., 2006), Senna obtusifolia L. (Norsworthy and Oliveira, 2006) and B. tourefortii (Chauhan and Johnson, 2010).
Acknowledgements
This work was carried out with the support of the “Cooperative Program for Agriculture Science & Technology Development (Project No. PJ01347901901)” Rural Development Administration, Republic of Korea and BK21 plus program through the National Research Foundation (NRF) funded by the Ministry of Education of Korea.
Authors Information
Kee Woong Park, Department of Crop Science, Chungnam National Univesrity, Professor
Jung Sup Choi, Korea Research Institute of ChemicalT echnology, Researcher
Botir Khaitov, Department of Crop Science, Chungnam National University, Researcher
Aung BoBo, Department of Crop Science, Chungnam National Univesrity, Ph.D. student
WeiQiang Jia, Department of Crop Science, Chungnam National University, Researcher
Le Thi Hien, Department of Crop Science, Chungnam National University, Ph.D. student
Mirjalol Umurzokov, Department of Crop Science, Chungnam National University, Master student
Farrukh Ruziev, Department of Crop Science, Chungnam National University, Master student
In Kon Park, Syngenta Korea, Researcher