Introduction
Cerastium glomeratum is a native plant of Europe and was introduced in Japan about a century ago (Asai et al., 2012). C. glomeratum has been reported as a cadmium and chromium accumulator plant and is useful for phytoremediation process in heavy metal contaminated soil (Sajad et al., 2020; Xia et al., 2018). C. glomeratum is also reported for antibacterial activity (Ullah et al., 2020) and consist several secondary metabolites like glycolipids in its glandular trichome that protects the plants from herbivores and pathogens (Asai et al., 2012). However, It has been reported as a green bridge, serving as a vector in the cross-transportation of Tomato chlorosis virus (Kil et al., 2015). Moreover, C. glomeratum is one of the dominant exotic weed on Korean peninsula and is considered threat for Korean agro-ecosystem (Park et al., 2020).
Research on weeds has attracted much interest in recent years (Bai, 2014; Pimentel et al., 2000). Weeds are the successful colonizers of human-made habitats and grow in every crop fields, causing great loss in the yield due to competition and allelopathy (Qasem and Foy, 2001). The effects of weeds greatly vary as our activities, some hampering agriculture, some interfering with habitat management and societal infrastructure, and some even directly affecting human health (Darbyshire and Prasad, 2009). In the context of global trade, the dispersal of weeds from one region to another has had serious impacts on ecology and mankind (Bagavathiannan et al., 2019; Bai, 2014; Pimentel et al., 2000).
The management of exotic weed plants in view of climate change and ecological aspects has been challenging for sustainable agriculture production (Lim et al., 2009). Exotic weed plants have been a major problem due to their translocation to new areas (Oh et al., 2003). They have immense potentials for rapid habitat expansion and adaptation (Clements and Ditommaso, 2011). In addition, they rapidly invade agricultural lands, thereby disrupting agricultural productivity (Mohler, 2001; Nichols et al., 2015).
There is a continuous change in cropland agriculture due to the introduction of new genotypes and crop species (Clements et al., 2004). Exotic species pose serious ecological and financial threats. The United States alone annually spend US$ 40 billion to avert invasive organisms. Of this amount, US$ 26.4 billion is estimated to be spent on the management of cropland weeds (Clements et al., 2004). The government of South Korea invests an extensive budget for invasive plant management but is deficient in managing weeds such as Cerastium glomeratum, which has been a great threat to the maintenance of agriculture in South Korea. It has been reported that Cerastium glomeratum is highly dominant in Gyeonggi, Gangwon, and Jeju Provinces of South Korea (Oh et al., 2004).
Available data suggest that approximately 321 species of exotic plants have adapted South Korea, and approximately 100 of these exotic weeds have inflowed since 2009 (Park, 2009). The native origins of these plants have been reported to be mostly from tropical America, Eurasia, North America, and Europe. The taxonomical hierarchy of naturalized exotic plants in Korea represents 39 families, 174 genus and 321 species (NKISBS, 2020).
Due to the increase in international trade, the number of exotic plants in South Korea has been increasing. The exotic plants impair the natural flora and fauna in various ways such as pest and invasive species (Orapa, 2001). For example, the common ragweed (Ambroisa artemisifolia L) contains allergens in its pollen, and the common ragweed and giant ragweed assimilate nutrients and secrete allochemicals, thereby inhibiting the vegetation and plant growth in their surroundings (Ko et al., 2019). Moreover, the invasion of exotic weed species may destroy the native species and force them for extinction (Adebayo and Uyi, 2010).
Management of weed in an agrarian land has been a major challenge for optimum and quality crop yield. Since, sustainable weed management is crucial for optimum crop yield, the appropriate selection of herbicides and its application doses is crucial to protect the environmental hazards and health (Hasanuzzaman et al., 2020). Hence, the current research is focused on identifying the different herbicides effect on the control rate of C. glomeratum through understanding its germination characteristics. Moreover, our research would help to predict the environmental condition required for the germination of C. glomeratum and efficient types of herbicides with appropriate doses required to inhibit its growth and distribution in Korea.
Materials and Methods
Plant materials
C. glomeratum seeds were collected on July, 2018, from the plum orchard located in Uiseong-gun, Gyeongsangbuk-do, South Korea (N36°21'38.1"E128°39'49.4"). The seeds were stored at a low temperature (4℃) for one month and stored at room temperature (25℃) before being used for the experiments.
Growth response of C. glomeratum nder various light and temperature conditions
To investigate the characteristics of seed germination under different light conditions and temperature, 10 seeds were sowed on a disposable sterile Petri dish (58×17 mm) supplied with filter paper. The temperature of the incubator was set as 5, 15, 25, and 35℃ (BioFREE, BF-50SIR). Distilled water (1 mL) was supplied at intervals of 3 days to retain the moisture. Dark-condition groups were fully covered by an aluminum foil to block light. The germination rates were assessed 3, 5, 7, 9, 15, and 25 days after sowing (DAS). Germination was regarded as having a radicle >2 mm. Plant length was measured at 25 DAS.
Growth response of C. glomeratum in different sowing depths
To investigate the characteristics of seed germination in different seeding depths, 20 seeds were sowed in a pot filled with horticultural soil (coco-peat 65-70%, peat-moss 8-12%, vermiculite 10-14%, zeolite 3-5%, pearlite 5-8%, water-soluble fertilizer, antimicrobials, and wetting agent) in a greenhouse with a temperature ranging 25±5℃. Seeding depths were 0 (surface sowing), 1, 2, 3, 4, and 5 cm. The germination rate was calculated at 13, 15, 20, and 25 DAS. Shoot and root lengths were measured at 25 DAS.
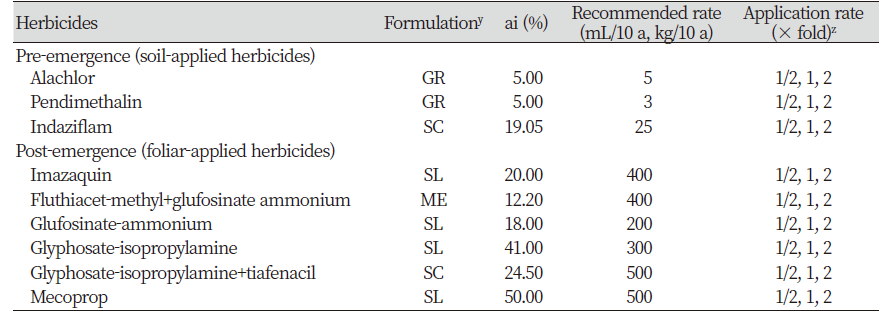
Herbicide selection
Pre-emergence (Soil applied herbicides) treatment
To investigate the management of C. glomeratum by herbicide treatments of the soil, 20 seeds were sowed in a plastic pot and covered with soil for up to approximately 1 cm. Three days after sowing, the pots were treated with a herbicide (alachlor, indaziflam and pendimethalin) through a sprayer. After 14 days of herbicide application, shoot length and dry weight were measured, and control values were calculated Formula 1. The shoot lengths were measured from the soil surface to the apex of the longest leaf. The germination rate was calculated by following Formula 2.
Formula 1. Control value (%)=(untreated group weight-treated group weight/untreated group weight)×100
Formula 2. Germination (%)=(total number of germinated seeds/total number of seeds per assay)×100
Post-emergence (foliar applied herbicides) treatment of herbicides
To investigate the control of C. glomeratum by foliar applications, seeds were germinated in a plastic tray. After 20 days, five equally sized seedlings were transplanted to plastic pots. 100 mL of herbicides was prepared in hand sanitizer bottle from each brand and sprayed after 10 days of transplantation. After 14 days of herbicide application, shoot lengths and dry weight was measured. The control values were calculated by following formula 1 as mentioned in above section. The herbicides used as pre-emergence and post-emergence with their formulation and application rate are mentioned in Table 1.
Statistical analysis
The study was carried out in a complete randomized design with three replications. Statistical analysis was performed with SAS 9.4 software (SAS Institute, Cary, NC, USA) and the mean values among the treatments were separated using Duncan’s multiple range test.
Results and Discussion
Effect of Light and temperature on C. glomeratum germination and growth
The germination rate was significantly affected by light and temperature. In the beginning, no germination was observed until 5 DAS, except for an 8% germination rate at 25℃. However, the seeds rapidly germinated at 15℃ at 7 DAS, resulting in a 45% germination rate at 25 DAS, which was the last day of the experiment. At 25℃, the germination rate increased over time to a certain extent, showing a 28% germination rate at 25 DAS. Other temperature conditions (5℃ and 35℃) showed a 0% germination rate. The shoot length showed a similar trend as the germination rate. At 25 DAS, the shoot length was 0.7 cm at 15℃, which was 40% longer than that (0.4 cm) at 25℃ (Fig. 1).
Effect of dark conditions in C. glomeratum germination and growth under various temperature
Under the dark conditions at 25℃, the initial germination appeared at 3 DAS, which is much earlier than that under the light conditions. 20% of the seeds were germinated at 5 DAS, and the germination rate continued to increase up to 28% at 7 DAS. Finally, the germination rate increased to 33% at 25 DAS. However, at 15℃, the first germination started at 7 DAS, which was later than the germination time at 25℃. At 25 DAS, the germination rates were 21.7% and 65.2% at 15℃ and 25℃, respectively. The shoot length was significantly increased by 17% at 15℃ compared with that at 25℃ (Fig. 2). The current results showed that the germination only appeared at 15℃ and 25℃ under both the light and dark conditions. The germination rates of the seeds at 15℃ and 25℃ at 25 DAS were 45.0% and 28.3%, respectively, under the light conditions, and 21.7% and 33.3% under the dark conditions. The shoot-length was 3-4 cm in the dark, but <1 cm in the light. However, the seedlings exhibited yellow leaves and under-developed roots and stems in the dark. The seedlings might be damaged by the etiolation resulting from continuous darkness.
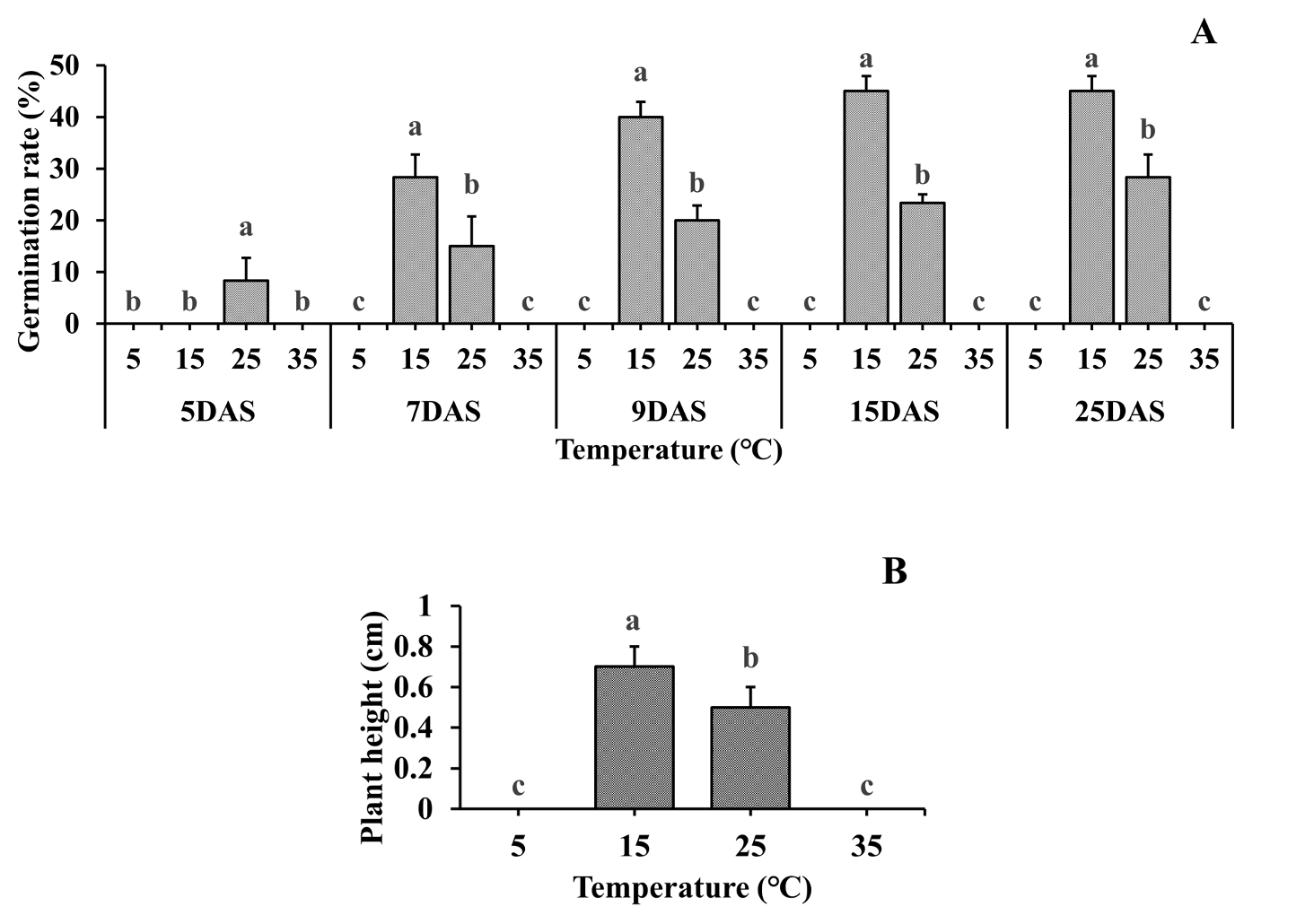
Fig. 1.Germination characteristics of C. glomeratum at various temperature under light. (A) Germination rate and (B) shoot length. Error bars represent standard deviations. Each data point represents the mean of at least six replications. DAS: Days after sowing. a-c: Bars with different letters are significantly different from each other at P≤ 0.05.
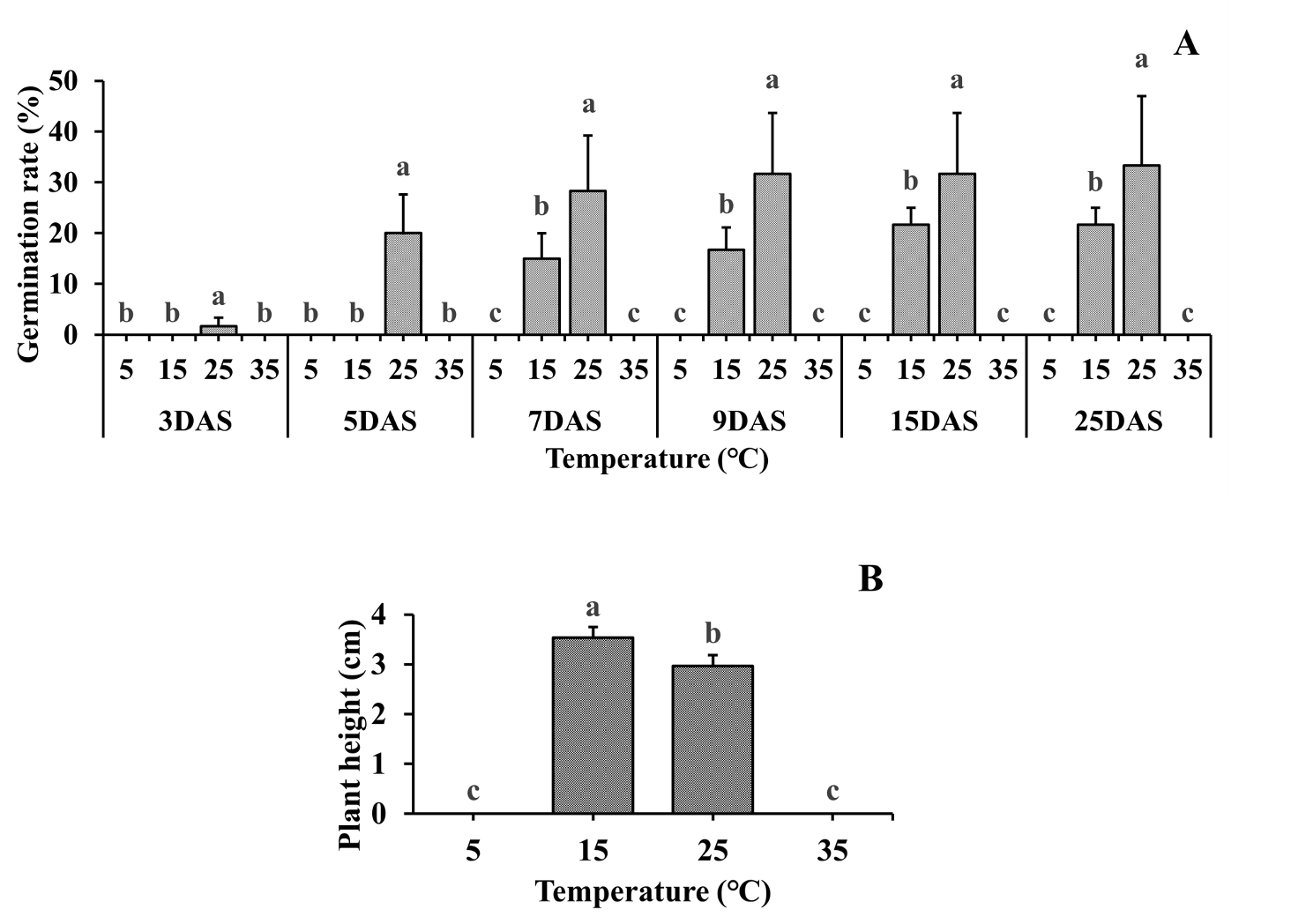
Fig. 2.Germination characteristics of C. glomeratum at various temperature under dark condition. (A) Germination characteristics and (B) shoot length. Error bars represent standard deviations. Each data point represents the mean of at least six replications. DAS: Days after sowing. a-c: Bars with different letters are significantly different from each other at P≤0.05.
Germination characteristics of sticky mouse-ear chickweed in different sowing depths
The seeds of sticky mouse-ear chickweed were micro-capped with a diameter of ~1 mm to ensure that their germination rates could be affected by their seedling depths. After 13 days of sowing, the seedlings began to emerge, and the most frequent at 1 cm depth which was 33.3% at 13 DAS, 41.6% at 15 DAS, 48.3% at 20 DAS, and 53.3% at 25 DAS. The threshold depth level for seed germination was considered 4 cm as no germination was observed at 5-cm depth (Fig. 3). The shoot and root lengths were higher at plants grown under 1 cm depth. They reached the longest length at 25 DAS when the seeds were sown at 1-cm depth and their lengths gradually decreased as the depth of sowing increased.
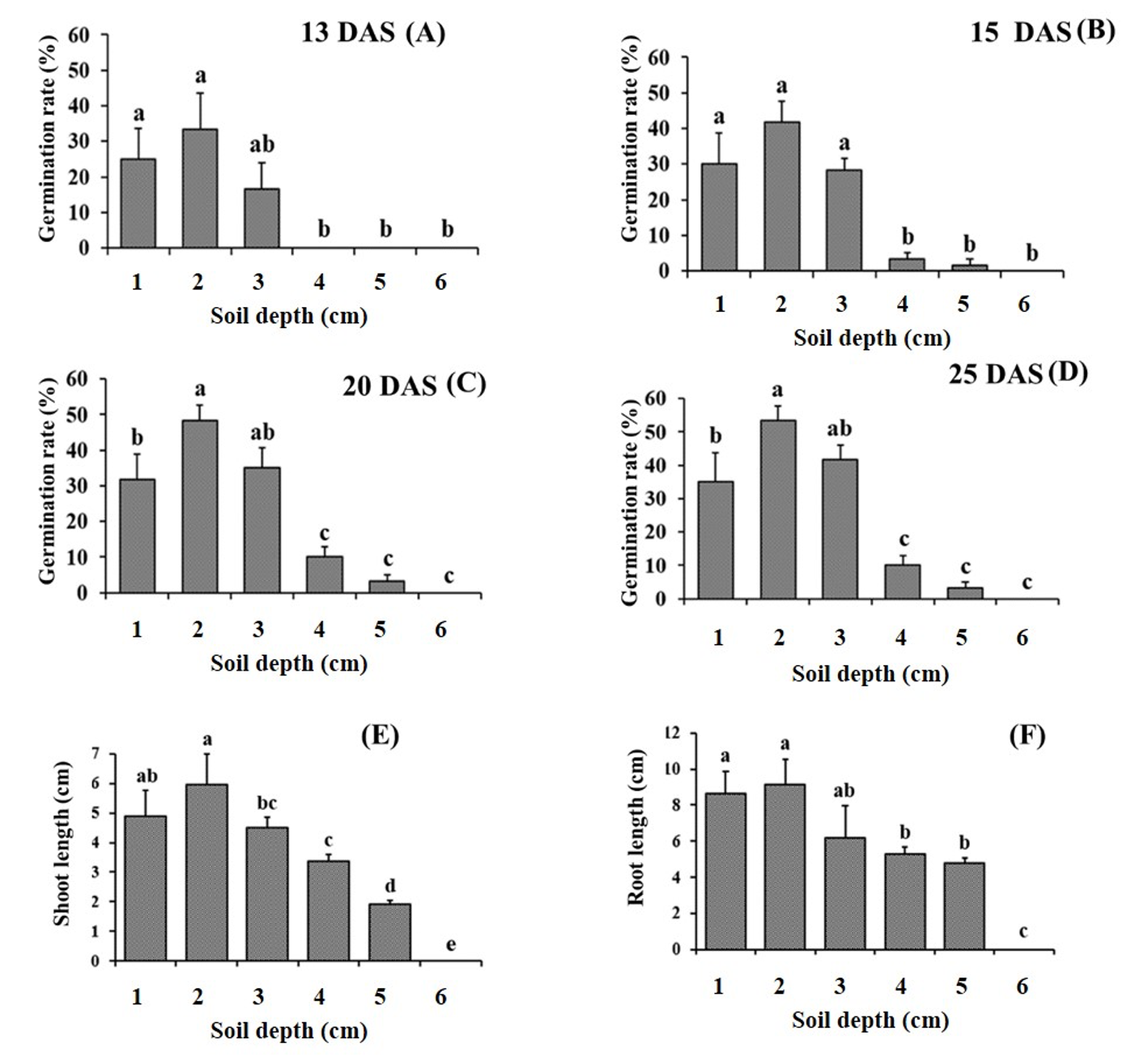
Fig. 3.Effect of various sowing depths on the germination and growth characteristics of C. glomeratum. (A, B, C and D) Germination rate, (E) shoot length and (F) root length. Error bars represent standard deviations. Each data point represents the mean of at least six replications. DAS: Days after sowing. a-e: Bars with different letters are significantly different from each other at P≤0.05.
Effect of herbicides on sticky mouse-ear chickweed occurrence and growth
Effect of soil-treated herbicides
Our results showed that the higher germination inhibition effect by herbicides followed the trend of indaziflam>alachlor>pendimethalin, especially the indaziflam 2-fold group showed 0% of germination rate.1/2-fold treatment groups also exhibited a declined germination rates with all types of herbicides. In the case of alachlor treatment, the control value was lower than that of other herbicide treatments. The soil-treated herbicides were aimed to inhibit germination for pre-occurrence management of weeds and had shown the best effect even at 1/2 doses. Indaziflam treatment had the best effect on the prevention of the germination of sticky mouse-ear chickweed since the germination was 100% inhibited by 2-fold treatment (Fig. 4 and Fig. 5). However, Pendimethalin 1/2 dose application has shown similar result compared to pendimethalin two-fold application. Moreover, 1/2 dose of pendimethalin has similar results when compared to two-fold of alachlor. Therefore, two fold indaziflam or 1/2 dose of pendimethalin appears to be the most suitable herbicide to avert sticky mouse-ear chickweed.
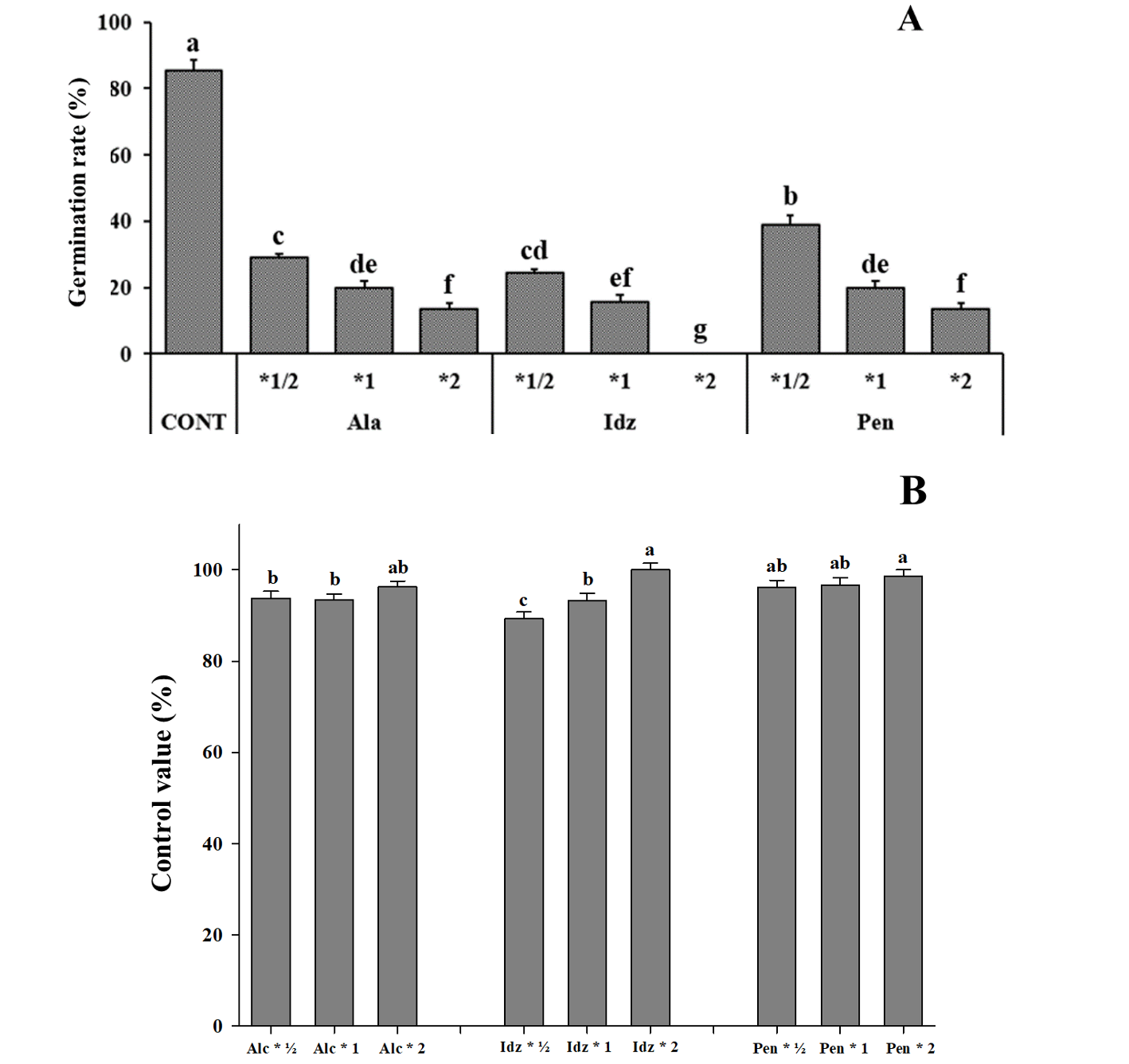
Fig. 5.Effect of soil herbicide treatment on C. glomeratum, growth, and control at 14 DAT. (A) Germination rate and (B) control rate. Error bars represent standard deviations. Each data point represents the mean of at least six replications. Cont: Control; Alc: Alachlor; Idz: Indaziflam; Pen: Pendimethalin. a-g: Bars with different letters are significantly different from each other at P≤0.05.
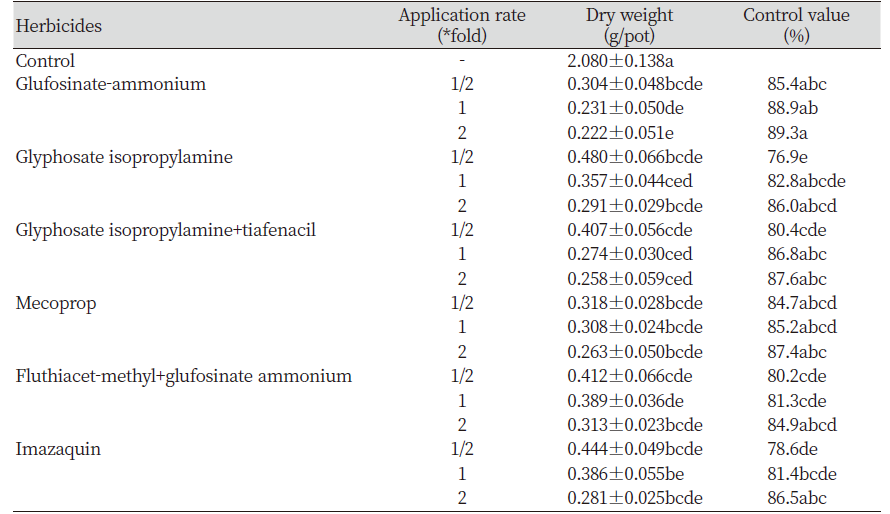
Effect of foliar-treated herbicides
Most of the herbicides showed approximately 80% of the control rate on foliar application (Table 2). Glufosinate ammonium, glyphosate-isopropylamine, tiafenacil, mecoprop, and flutiathe-methyl treatment groups showed > 80% of the control value even at the 1/2-fold treatment. Therefore, it can be considered that sticky chick mouse-ear chickweed management can be possible even with low-dose foliar herbicide treatments.
Discussion
The climatic condition of a particular area is a crucial factor for the vegetation of the plants species. The Korea lies in between Chinese and Japanese island and is influenced with the Asian monsoon its average annual mean temperature ranges 16.6℃ (summer) to 6.6℃ (winter), however, the temperature may fluctuate below 0℃ during Dec-March. Our results showed that C. glomeratum thrives better at 15℃, there is higher possibility of the occurrence and distribution in pre-winter (October-November) in South Korean land. Our results are in line with (Kim et al., 2018b) who showed the optimum germination of exotic weed species-common groundsel (Senecio vulgaris) at 15℃ to 20℃. Moreover, the seed emergence pattern and growth are also affected by rainfall pattern and distribution might be affected through extreme weather conditions like flood and typhoon.
The selection of herbicides types and its minimal application dose to control weed incidence is crucial for sustainable agriculture. Our results showed that Pendimethalin with 1/2 dose application and Indaziflam with two-fold application could completely suppress the emergence of C. glomeratum as pre-emergence treatment. Similarly, as a post-emergence treatment on foliar application of herbicides glufosinate ammonium has maximum control rate with minimum doses of application. Despite the beneficiaries of herbicides application to control the weed incidence and distribution, there are several other environmental hazardous factor influence by the excessive use of herbicides (Hasan and Ansari, 2016; Way, 1969). Kim et al. (2018a) reported the harmful effect of pendimethalin and alachlor application for inhibiting the growth of Platycodon grandiflorum. Moreover, the application of herbicides may cause phytotoxicity in beneficial crops and affect the ecological system, thus, broad research and survey is required prior to the application.
Conclusion
Based on these results, it can be concluded that the seeds thrive better at 15-25℃ with excellent performance at15℃ and 1 cm soil depth. For 100% pre-emergence control, we recommend
pendimethalin application at the 2-fold dose recommended for weeds. Glufosinate ammonium or mecoprop at 2-fold recommended doses results in the best post-emergence control with a rate of 89%. Since even the half-fold doses had >80% control rate, we recommend selecting the lower dose to minimize the hazardous effect of herbicides on the ecosystem.
Acknowledgment
This study was supported by Agenda Program (Project No. PJ013216032019), Rural Development Administration, Republic of Korea.
Authors Information
Chang-Wook Park, Department of Applied Biosciences, Kyungpook National University, Doctor of Philosophy
Arjun Adhikari, Department of Applied Biosciences, Kyungpook National University, Doctor of Philosophy
Eun-Jung Park, Division of Plant Biosciences, Kyungpook National University, Researcher
Sang-Mo Kang, http://orcid.org/0000-0002-9064-0006
In-Jung Lee, http://orcid.org/0000-0001-7154-4820