Introduction
Exotic weeds are plants that have intentionally or unintentionally left their place of origin and settled on another land by humans (Kim et al, 2018). Due to the recent increase in international trade, many foreign weeds have been introduced into Korea (Park et al., 2020a). Exotic weeds are a major risk factor for biodiversity because of their rapid spread within plant communities and their ability to displace native plants (Szymura & Szymura, 2016). In addition, interest in exotic weeds research is increasing recently because it disturbs agriculture and forestry and affects the surrounding ecosystem (Bai, 2014). Grassland is an agricultural land composed of perennial grasses used as forage and has multiple functions such as soil conservation, water purification, and supply habitat for wild animals (Lee et al., 2003). Exotic weeds that occur in these pastures lower the productivity and quality of forage, resulting in difficulties in the production of high-quality forage (Kim et al., 2003). Tall fescue (Festuca arundinacea) is a perennial grass, an ecologically important grassland crop in temperate northern hemisphere regions (Shahabzadeh et al., 2020). Its tolerance to drought, salinity, heavy metals, and overgrazing and its ability to minimize soil erosion are well known (Kim et al., 2022). White clover (Trifolium repens) is a widely distributed grassland crop worldwide from the northern tropical mountains to temperate regions (Gibson & Cope, 1985) and is a major leguminous crop in temperate pastures, with known benefits in terms of nitrogen fixation and grassland quality (Sheaffer & Marten, 2010). Solidago altissma. L is a perennial weed in the Asteraceae family, native to North America, and is a weed spreading throughout the world, including Europe and Asia (Weber, 2000). In Korea, it was introduced as beekeeping and ornamental plant before 1970, and has since spread to the southern part of the Korean Peninsula (Park et al., 2020b). The above-ground part grows to about 2.5 m, and one plant can produce about 20,000 seeds (Kato-Noguchi and Kato, 2022). It spreads to surrounding areas based on such high seed yield and vegetative propagation ability using rhizomes and allelopathy (Johnson et al., 2010; Nishidono and Tanaka, 2022). Due to these characteristics of S. altissima, it was designated as an ecosystem-disturbing organism by the Ministry of Environment in 2009. Allelopathy is defined as the release of secondary compounds, called allelochemicals, from one species into the surrounding environment, with harmful or beneficial effects on the germination, growth, and development of other plant species (Li et al., 2010). Among the allelochemicals isolated and identified from S. altissima, cis - dehydromatricaria ester (cis - DME) has been reported to impose toxicity on other species (Kobayashi et al., 1976). However, studies on the effect of S. altissima and allelopathy on grassland crops are limited in Korea. Therefore, this study was carried out to determine the effect of the root powder and MeOH extract of S. altissima on the germination and seedling growth of forage crops.
Materials and Methods
Plant material
S. altissima was collected from 1240-1 Panmun-dong (35°10'16.96''N, 128°2'32.10''E) Jinju-si, Korea. It was separated into the shoot and root, dried under the shade, and homogenized with homogenizer (A11BS0A0, IKA, Staufen, Germany). While the powder was stored in a 4℃, methanol (MeOH) extraction, seed bioassay, and germination experiment by mixing ratio of powder and soil were carried out. Tall fescue seeds were purchased from Danong (Kentucky 31 tall fescue, Da- nong Inc., Namyangju, Korea) and white clover seeds were purchased from Ubiqsolution (White clover, Ubiqsolution, Guri, Korea) and used in the experiment.
MeOH extraction
Shoot and root powders of S. altissima 50 g were placed in a 1 L beaker, 500 mL MeOH was added, and extracted 3 times at 500 rpm using a stirrer (MSH-20D, DAIHAN Scientific Inc., Wonju-si, Korea) at intervals of 24 hours and each extract was mixed. After that, it was concentrated in a 500 mL recovery flask using a rotary vacuum evaporator (Eyela Rotary Vacuum Evaporator NN series, Eyela, Tokyo, Japan). 100 mL of d-H2O was dispensed in a concentrated flask to dissolve the extract. After dispensing 20 mL of MeOH extract solution into conical tubes (50 mL, SPL Life Science, Pocheon, Korea). It was freezed using a deep-freezer (IU2386D, Thermo Fisher Scientific, Marietta, OH, United States) and dried for seven days using a freeze dryer (PVTFD20R, Ilshin Inc., Seoul, Korea). MeOH extraction dried product was stored in -80℃ deep-freezer and used for the experiment.
In vitro seed bioassay
The in vitro seed bioassay was performed to confirm the effect of S. altissima shoot and root MeOH extracts on the germination of forage crops. A stock solution of 10,000 mg L-1 concentration was prepared by dissolving the MeOH extract dried product in d-H2O, and then a MeOH solution according to concentration was prepared up to a minimum of 156.25 mg L-1 by serial dilution. A filter paper (Advantec no. 2, Toyo Roshi Kaisha Ltd., Tokyo, Japan) was laid on a Petri dish (60 × 15 mm, SPL Life Science, Pocheon, Korea) and 2 mL of each concentration solution was dispensed to prepare a seed medium. After surface sterilization of F. arundinacea and T. repens seeds with 1% sodium hypochlorite, they were rinsed with distilled water 10 times to complete sterilization. Put twenty seeds in each petri dish and seal it. After sealing, it was germinated for 7 days in a plant growth chamber (JSPC-420C, JSR Corporation, Gongju, Korea) with a temperature of 25℃, relative humidity of 60%, a light intensity of 6,850 lux, and a photoperiod of 16/8 (day/night). A treatment-dose curve was calculated by measuring the fresh weight of the forage crop seedlings, and the dose that capable of inhibiting the growth of plants by 50% (GR50, growth retardation 50%) was obtained using this. This experiment was conducted in five repetitions of the completely random arrangement method.
Effect of the mixing ratio of S.altissima root powder and soil on seedling growth of forage crops
The experiment was carried out according to the method of Park et al., (2015). This experiment was carried out in the glass greenhouse attached to the College of Agriculture and Life Sciences, Kyungpook National University, and was performed in 3 repetitions of the completely random arrangement method. The soil was horticulture soil (Hanareum, Shinsung mineral, Goesan, Korea). To evaluate the growth inhibition of S. altissima root powder to forage crops, set the mixing ratio of root powder and horticulture soil (root powder: soil, w /w) 20% (20 : 80), 40% (40 : 60), 60% (60 : 40), 80% (80 : 20). Each was mixed and used in the experiment. A round pot (9 cm × 10 cm) was filled with the soil by 5 cm, and after sowing 20 forage seeds, each powder mixed with soil was covered by 2 cm. Three weeks after sowing, shoot length, root length, fresh weight, and dry weight of forage crops were measured.
Root extract foliage treatment
To confirm the effect of MeOH extract on the forage crop seedling growth, the root extract foliage treatment was performed. This experiment was carried out in the glass greenhouse attached to the College of Agriculture and Life Sciences, Kyungpook National University and was performed in three repetitions of the completely random arrangement method. After dissolving the MeOH extract in d-H2O to prepare a stock solution with a concentration of 100,000 mg L-1, serial dilution was conducted by adding d-H2O to a minimum concentration of 6,250 mg L-1. For forage crops, 5 cm of soil was filled in a round pot (9 cm × 10 cm), twenty seeds of F. arundinacea and T. repens were sown each and the soil was covered 2 cm thick. Three weeks after sowing, an electrodepositing agent Tween 20, (Duksan Genetal Science Inc., Seoul, Korea) at a concentration of 0.1% was added to 5 mL of each MeOH solution, and foliar treatment was performed three times at intervals of one week. After the third foliar treatment, shoot length, root length, fresh weight, dry weight, and chlorophyll content of forage crops were measured. The chlorophyll content of the leaves was measured using a portable CCM-300 chlorophyll content meter (ADC BioScientific Ltd., Herts, England).
Statistical analysis
All experimental data were analyzed using ANOVA and Duncan's multiple range test (p<0.05) to examine the significant differences between the mean values. It was run using SAS statistical software (version 9.4, SAS Institute, Cary, NC, United States).
Results and Discussion
Effect of the mixing ratio of S. altissima root powder and soil on seedling growth of forage crops
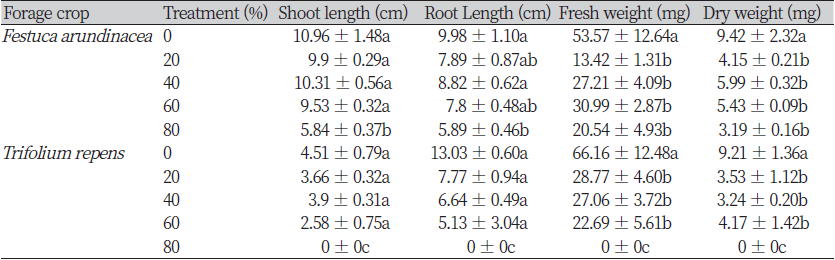
The result of the growth survey of seedlings according to the mixing ratio of S. altissima root powder and soil showed no significant difference in the shoot and root length of F. arundiancea in 20%, 40%, and 60% treated groups when compared to the untreated group. However, in 80% treatment group, the shoot and root length were significantly decreased by 47% compared to the untreated group. Fresh weight and dry weight were significantly decreased by 41% in all treated groups (20%, 40%, 60%, 80%) compared to untreated groups. In the 20% treatment group, the fresh weight decreased the most to 74.95% and in the 80% treatment group, the dry weight decreased the most to 66.13% compared to the untreated group (Table 1).
In addition, growth survey of T. repens, shoot and root length did not show a significant difference in the 20%, 40%, and 60% treated groups compared to the untreated group. Unlike the previous F. arundinacea, 80% treated group did not germinate. In the case of fresh weight and dry weight, all treated groups showed significantly reduced values compared to the untreated group. In the 60% treatment group, the fresh weight showed the most decreased value of 65.7%, and in the 80% treatment group, the dry weight decreased the most to 64.82%. Unlike the in vitro seed bioassay, which will be described later, the germination rate of F. arundinacea is higher than T. repens. Because F. arundinacea, which belongs to the Poaceae family, is an underground germination type and T. repens, which belongs to the Fabaceae family, is a ground germination type (Sheaffer & Marten, 2010). Accordingly, F. arundinacea was able to germinate through a hard mixture of root powder and soil using cotyledon. However, it is thought that T. repens could not germinate because it did not use the cotyledon.
Herbicidal activity of MeOH extract from S.altissima root on forage crops
As a result of a seed bioassay to confirm the effect of MeOH extract from the S. altissima root on the germination of forage crops, the MeOH extract has a significant inhibitory effect on the growth of forage crop seedlings. When treated with F. arundinacea seeds, the fresh weight of the seedlings was inhibited from the 312.5 mg L-1 treatment group and the fresh weight decreased by 95% in the 10,000 mg L-1 treatment group compared to the untreated group. When treated with T. repens seeds, the fresh weight was slightly increased in the 312.5 mg L-1 treatment group, but the fresh weight decreased from the 625 mg L-1 treatment group and the fresh weight decreased by 88.1% in the 10,000 mg L-1 treatment group compared to the untreated group. The trend line according to the concentration of MeOH extract in the underground part of S. altissima, the GR50 value was 1,034.78 mg L-1 when treated with F. arundinacea and was 2,608.1 mg L-1 when treated with T. repens. The trend line and equation for each forage crop are shown in Fig. 1 and Fig. 2. Based on this, it is thought that the MeOH extract from the S.altissima root has a greater germination inhibitory effect on the F. arundinacea, a Poaceae family forage crop.
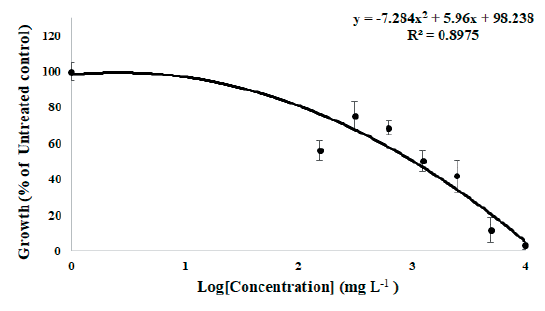
Fig. 1.Growth retardation curve of F. arundinacea seedling fresh weight by treatment of S. altissima root MeOH extract in vitro seed bioassay. The regression is y=-7.2484x2 -5.96x+98.238 and the calculated growth retardation 50% (GR50) value of MeOH extracts of S. altissima root to F. arundinacea is 1,034.78 mg L-1.
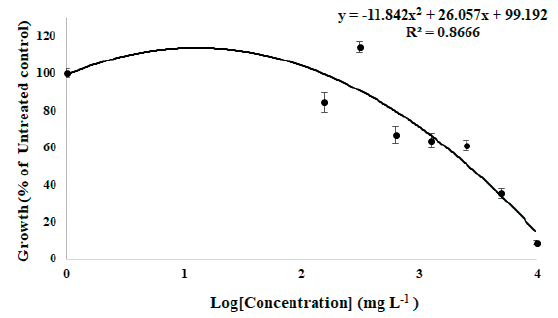
Fig. 2.Growth retardation curve of T. repens seedling fresh weight by treatment of S. altissima root MeOH extract in vitro seed bioassay. The regression is y=-11.842x2 + 26.057x + 99.192 and the calculated growth retardation 50% (GR50) value of MeOH extracts of S. altissima root to T. repens is 2,608.10 mg L-1.
Effect of foliage treatment of MeOH extract from S. altissima root on the growth of seedlings of forage crops
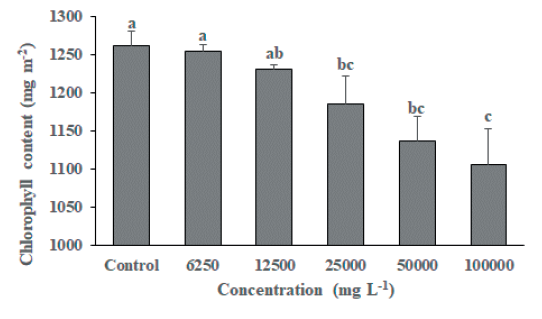
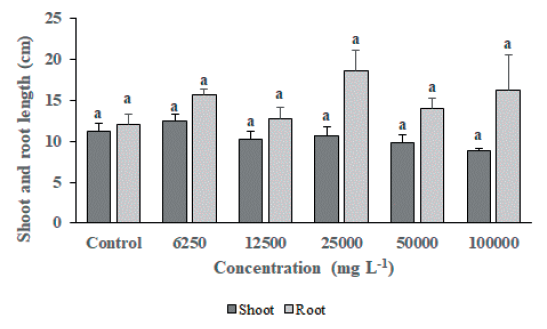
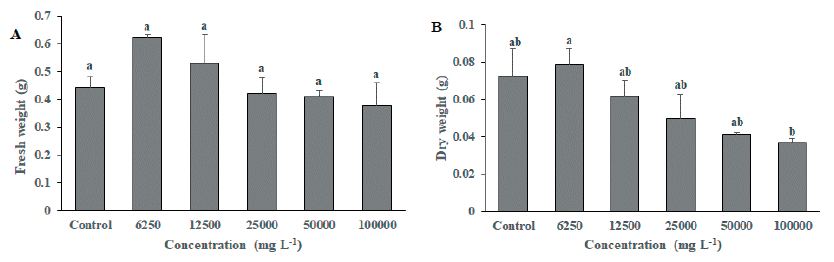
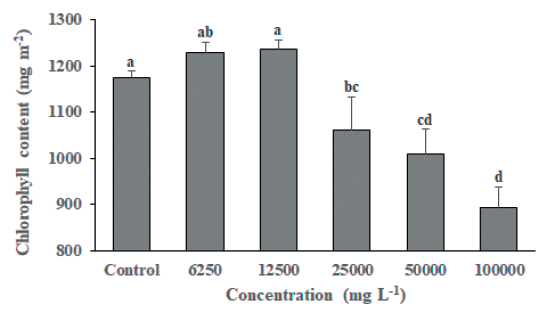
As a result of foliage treatment of MeOH extract from S. altissima root extract, F. arundinacea did not show a significant difference between the untreated group and the MeOH extract treated group by concentration in the growth survey (shoot length, root length, fresh weight, and dry weight), but the chlorophyll content of 25,000 mg L-1, 50,000 mg L-1 and 100,000 mg L-1 treatment groups were significantly decreased compared to the untreated group (Fig. 3). On the other hand, T. repens growth survey results (Fig. 4 and Fig. 5) showed that shoot length, fresh weight, and dry weight were significantly reduced in the 100,000 mg L-1 treatment group compared to the untreated group. It was confirmed that the chlorophyll content was significantly reduced in the 25,000 mg L-1, 50,000 mg L-1, and 100,000 mg L-1 treated groups compared to the untreated group (Fig. 6). This is considered to be due to the characteristics of F. arundinacea that can grow under various stress conditions (Kim et al., 2022).
In this study, Solidago altissima, an ecosystem-disturbing plant, was extracted with methanol, and it was confirmed that the extract had an effect on the growth of forage crops. In the in vitro seed bioassay experiment, the MeOH extract of S. altissima inhibited the growth of tall fescue and white clover seedlings. This result suggests that influx of S. altissima into the pasture, it may adversely affect the cultivation and quality of forage crops. Some ecological disturbance organisms such as carolina horsenettle (Nichols et al., 1991) and ragweed (Rosenbaum et al., 2011) affecting the quality of forage crop, S. altissima can also affect the quality of forage crop. However, since the experiment was conducted on only tall fescue and white clover, it is considered that the effect should be evaluated on other forage crops. Based on the results of the foliage treatment experiment, tall fescue suffered relatively little damage compared to white clover, it can be expected that the S. altissima extract has a greater herbicidal effect on Fabaceae family forage crops. However, since the degree of damage may vary depending on the treatment period and growth conditions, it is considered that additional research on this should be conducted.
Although the information on the extract of S. altissima is limited, the studies on the lettuce growth inhibitory activity of S.altissima root n-hexane extract have been conducted (Nishidono and Tanaka, 2022). In a study on the phytochemicals of S. altissima, it was known that the S. altissima extract contains polyacetylene (Ichihara et al., 1976), monoterpene and sesquiterpene (Johnson et al., 2010), diterpene (Nishino et al., 1984; Bohlmann et al., 1985), triterpene (Okano et al., 1983), flavonoid glycosides (Wu et al., 2007), polyphenols (Jin et al., 2008). Among them, cis-dehydromatricaria ester (cis-DME) is a substance that has been confirmed to be able to inhibit germination and growth of plants at a level of 1 to 20 ppm (Ichihara et al., 1978; Uesugi and Kessler, 2013). It is believed that the methanol extract of S. altissima, which was extracted in this study, may contained cis-DME, affect the germination and seedling growth of forage crops. However, in order to identify the exact cause, the process of separating and purifying is required, and bioassay on forage crop using the identified substances are also required. In this study, it was confirmed that the S. altissima root powder and methanol extract had an allelopathic potential on the forage crops. This result can be used as basic data for future research on S. altissima. Thereafter, separation and purification, quantitative and qualitative analysis through instrumental analysis is required.
Conclusion
In conclusion, the S. altissima extract application has adverse effect on the morphological attributes including germination of T. repens and F. arundinacea. Hence it could be predicted that S. altissima has significant potential for dominance as well as inducing phytotoxicity over forage crops. The current experiment could lay out a basic foundation for understanding the probable risk of S. altissima exposure on Korean pasture land and formulating strategies for its efficient management.
Acknowledgment
This study was supported by the Agenda Program (Project No. PJ 015026022022) Rural Development Administration, Republic of Korea.
Authors Information
Ho-Jun Gam, Department of Applied Biosciences, Kyungpook National University, Master student
Yosep Kang, Department of Applied Biosciences, Kyungpook National University, Master student
Eun-Jung Park, Department of Applied Biosciences, Kyungpook National University, Researcher
Sang-Mo Kang, Department of Applied Biosciences, Kyungpook National University, Doctor of Philosophy
Ki-Yong Kim, National Institute of Animal Science, RDA, Researcher
In-Jung Lee, http://orcid.org/0000-0001-7154-4820