Introduction
In Korea, there are a total of 28 families and 166 species of exotic weeds in agricultural lands such as paddy fields, fields, orchards, and pastures, and among them, 23 families and 80 species of exotic weeds in pastures are occupied (Kim et al., 2018). Rumex acetosella (Red sorrel) is an exotic weed distributed nationwide (Lee et al., 2001). It is a weed with high dominance even in pastures and is a weed type with a high incidence if the management conditions of the pastures are poor (Lee et al., 2016). R. acetosella is one of 16 species of plants designated as ecological disturbance species and can be propagated by rhizomes and seeds. Through previous research on R. acetosella, it was possible to remove the shoot part of R. acetosella by foliage treatment with mecoprop, a selective herbicide, through the herbicide selection process for chemical control of R. acetosella, but it was not known whether the root part of R. acetosella was controlled (Kim et al., 1999), and the number of germinated populations from the land from which the shoot parts were removed after mecoprop treatment increased more than four times compared with the untreated group (Kim et al., 2003a). It is also known to be resistant to some herbicides, such as hexazinone (Li et al., 2014). Moreover, chrysophanic acid (Kim et al., 2003b) and catechol (Kim et al., 2004), herbicidal active substances isolated from R. acetosella, affect the surrounding pasture. There have been many experiments on R. acetosella so far, but no previous experiments have investigated the effects of treating R. acetosella shoots and roots on pasture plants. Therefore, in this study, the experiments were performed to investigate the effects of the shoot and root extracts on Festuca arundinacea (Tall fescue), Poaceae crop, and Trifolium repens (White clover), Fabaceae crop, representing pasture.
Materials and Methods
Plant Materials
R. acetosella used in the experiment was collected in May 2022 from Yugyum-ri, Gangdong-myeon, Gyeongju-si, Gyeongsangbuk-do (35˚59'15''N, 129˚16'34''E). It was freeze-dried using a freeze dryer (PVTFD20R, Ilshin Lab, Seoul, Korea). The freeze-dried sample was divided into the shoot and root parts and pulverized using a Homogenizer (29000A0, IKA, Staufen, Germany). F. arundinacea was purchased from Da-nong Inc (Kentucky 31 Tall Fescue, Da-nong Inc., Namyangju, Korea). T. repens was purchased from Dongguk Seedling Industry Inc (Dongguk Seedling White Clover Seed Landscape, Dongguk Seedling Industry Inc., Seoul, Korea).
Methanol extract
Put 500 g each of crushed R. acetosella shoot and root sample in a 2 L Erlenmeyer flask (FK10202000, Dongsung Science Inc., Gwangju, Korea) into 1 L MeOH and stir in a magnetic stirrer (MSH-20D, DAIHAN Scientific Inc., Wonju-si, Gangwon-do, Korea) for 24 hours. After that, the filter paper (Advantec No.2, Toyo Roshi Kaisha Inc., Tokyo, Japan) was laid on the Büchner funnel to obtain the methanol extract, and this process was repeated three times. The methanol extract was placed in a recovery flask and concentrated with a rotary vacuum evaporator (Eyela Rotary Vacuum Evaporator NN series, Eyela, Tokyo, Japan), and 100 mL of distilled water was dispensed. The sample was stored in a deep freezer (IU2386D, Thermo Fisher Scientific, Marietta, OH, United States) by aliquoting 20 mL into a conical tube (50 mL, SPL Life Science, Pocheon, Korea). Then, it was dried using a freeze dryer, and the dried sample was stored in a refrigerator at 4°C.
In vitro seed bioassay
An experiment was conducted to investigate the effect of R. acetosella shoot and root extracts on the germination of F. arundinacea and T. repens seeds. Seed bioassay was carried out with methanol extracts from the shoot and root parts of R. acetosella. First, sodium hypochlorite was dispensed in dH2O to sterilize the seeds of F. arundinacea and T. repens, and the sterilized seeds were placed 20 each on a petri-dish (60 mm × 15 mm, SPL Life Science, Pocheon, Korea) covered with filter paper. After preparing each 20,000 mg L-1 stock solution of the shoot and root extracts, serial dilution was performed. The concentrations used in the experiment were 20,000 mg L-1, 10,000 mg L-1, 5,000 mg L-1, 2,500 mg L-1, 1,250 mg L-1, 625 mg L-1. After dispensing 1 mL of methanol extract in a petri-dish with seeds, it was grown for seven days in a plant growth chamber (JSPC-420C, JSR Corporation, Gonju, Korea) with a temperature of 20°C, a humidity of 60%, and a light 6,850 lux, and the fresh weight was investigated. A dose-response curve was obtained based on the measured fresh weight, and the GR50 value, which is a dose that inhibits plant growth by 50% depending on the concentration of the extract, was calculated, and the experiment was repeated three times.
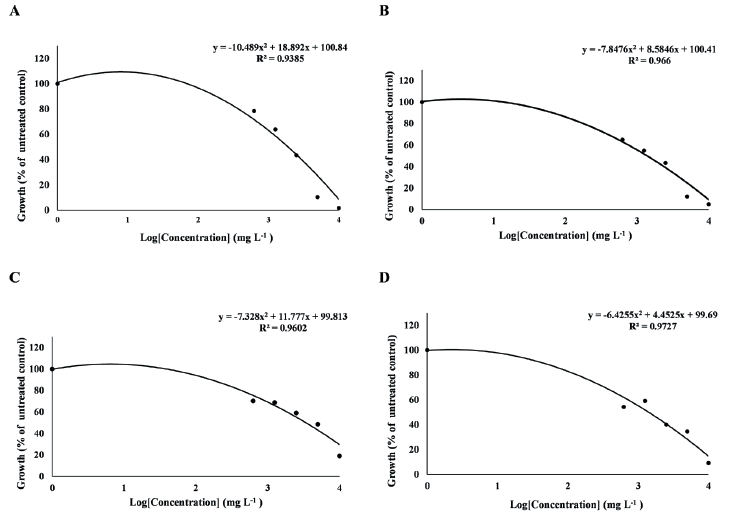
Fig. 1.The dose-response curve of T. repens and F. arundinacea treated with MeOH extract of R. acetosella. (A) T. repens treated with R. acetosella root extract,(B) T. repens treated with R. acetosella shoot extract, (C) F. arundinacea treated with R. acetosella root extract, (D)F. arundinacea treated with R. acetosella shoot extract.
Effect of R. acetosella shoot powder on F. arundinacea and T. repens growth
The experiment was carried out in the method described by Park et al. (2015) in a glass greenhouse. To investigate the effect of R. acetosella shoot powder on the growth of T. repens and F. arundinacea, the ratio of shoot powder to soil was set to 20:80, 40:60, 60:40, and 80:20. A pot (100 mm × 90 mm) was filled with 5 cm of soil, 20 T. repens and F. arundinacea seeds sterilized with sodium hypochlorite were sown each, and 2 cm of powder and soil mixtures were covered on the surface of the soil. Three weeks after sowing, the germination rate, shoot length, root length, fresh weight, and dry weight were investigated. The experiment was repeated three times.
Foliage treatment and chlorophyll content measurement
This experiment was conducted to confirm the effect of foliage treatment of R. acetosella shoot extract on F. arundinacea and T. repens in the glass greenhouse. After sterilizing the seeds in sodium hypochlorite solution, 20 seeds were sown in each pot (100 mm × 90 mm). After preparing 100,000 mg L-1 stock solution of the shoot extract, serial dilution was carried out and adjuvant Tween 20 (Duksan Genetal Science Inc., Seoul, Korea) was added. The concentrations used in the experiment were 100,000 mg L-1, 50,000 mg L-1, 25,000 mg L-1, 12,500 mg L-1, and 6,250 mg L-1. After four weeks of sowing, foliage treatment was performed three times with 5 mL at weekly intervals. Seven days after the last foliage treatment, shoot length, root length, fresh weight, and dry weight were investigated, and chlorophyll content was measured using a portable Chlorophyll Contents Meter (CCM-300, ADC Bioscientific Inc., Herts, UK). The experiment was repeated three times.
Statistical Analysis
All experiments were performed in three repetitions. A Statistics Analysis System (SAS Software, Version 9.4, SAS Institute Inc., CARY, NC, USA) program was used for statistical significance analysis. The difference between repetitions was tested at p<0.05 through Duncan’s Multiple Range Test (DMRT).
Results and Discussion
In vitro seed bioassay
As a result of obtaining the GR50 value, which is a dose that inhibits plant growth by 50% depending on the concentration of the extract, in F. arundinacea, the GR50 value of the root extract was 3,403 mg L-1 (y = -7.328x2 + 11.777x + 99.813, R2 = 0.9602), the GR50 value of the shoot extract was 1,409 mg L-1 (y = -6.4255x2 + 4.4525x + 99.69, R² = 0.9727). In T. repens, the GR50 value of the root extract was 1,902 mg L-1 (y = -10.489x2 + 18.892x + 100.84, R2 = 0.9385), the GR50 value of the shoot extract was 1,380 mg L-1 (y = -7.8476x2 + 8.5846x + 100.41, R2 = 0.966) (Fig. 1A-D). Both F. arundinacea and T. repens showed a lower GR50 value in the shoot extract than in the root extract.
Effect of R. acetosella shoot powder on F. arundinacea and T. repens growth
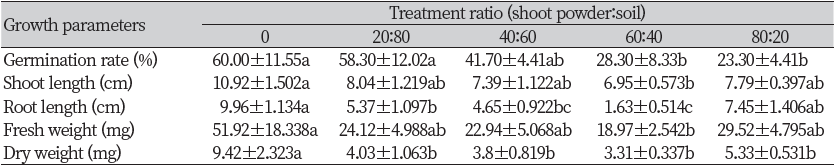
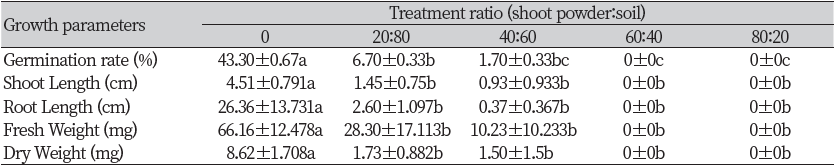
An additional greenhouse experiment was conducted on R. acetosella shoot parts, which showed a lower GR50 value in an In vitro seed bioassay. As a result of treating a mixture of the shoot extract and soil on the surface of the soil, the germination rate of F. arundinacea was significantly reduced compared with the control when the ratio of shoot powder was more than 60%. In the case of shoot length and fresh weight, the control showed a significant difference compared with the 60% treatment but did not show a significant difference with other treatments, and the control showed a significant difference with other treatments except for the 80% treatment in root length. Dry weight decreased significantly in all treatment groups compared with the control (Table 1).
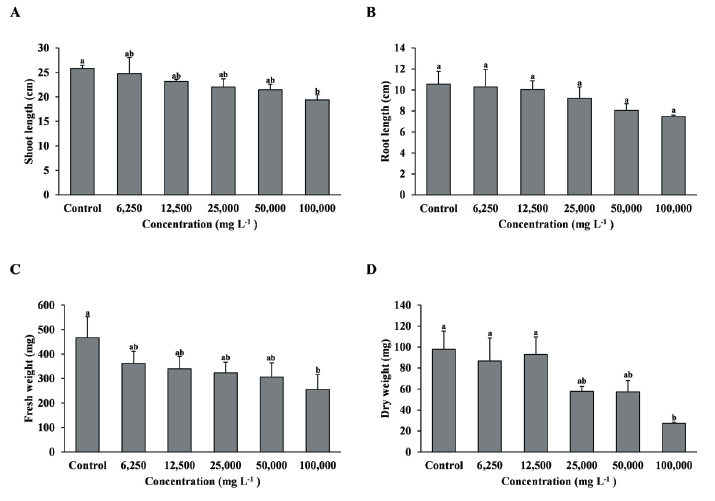
Fig. 2.Growth of F. arundinacea with foliage treatment by the concentration of extract of R. acetosella. (A) Shoot length, (B) Root length, (C) Fresh weight, (D) Dry weight. Each data point represents the mean of three replications. Error bars represent standard errors. Bars with different letters are significantly different from each other at p<0.05.
The germination rate of T. repens decreased as the ratio of the shoot powder increased. Shoot length, root length, fresh weight, and dry weight showed significantly lower values in all treatments compared with the control. Moreover, T. repens did not germinate in 60 and 80% of treatments (Table 2).
Foliage treatment and chlorophyll content measurement
As a result of foliage treatment of the shoot extract on F. arundinacea, the shoot length and fresh weight were significantly reduced when the 100,000 mg L-1 treatment was compared with the control, and dry weight also showed a tendency to decrease as the concentration of the extract increased, and the 100,000 mg L-1 treatment decreased significantly compared to the control, 6,250, and 12,500 mg L-1 treatments (Fig. 2A, C, D). However, root length was no significant difference between treatment groups (Fig. 2B).
As a result of foliage treatment of the shoot extract on T. repens, the shoot length was significantly decreased in the 100,000 mg L-1 treatment compared with the control, and fresh weight and dry weight were significantly decreased in the 50,000 and 100,000 mg L-1 treatments compared with the control and 6,250 and 12,500 mg L-1 treatments (Fig. 3A, C, D). However, root length was no significant difference between treatment groups (Fig. 3B).
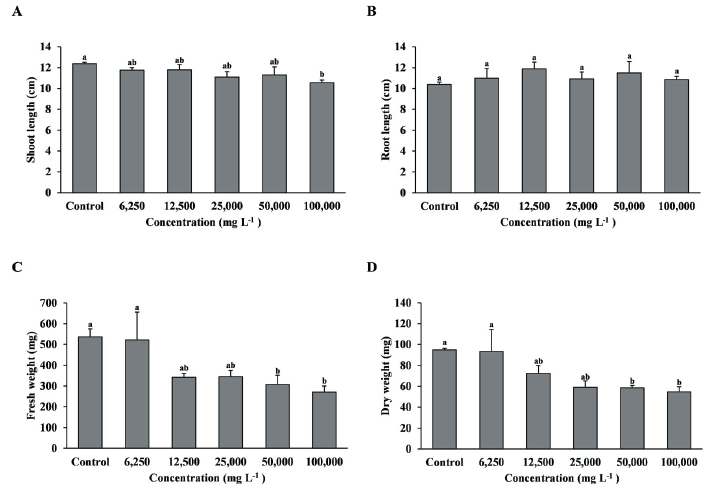
Fig. 3.Growth of T. repens with foliage treatment by the concentration of MeOH extract of R. acetosella shoot. (A) Shoot length, (B) Root length, (C) Fresh weight, (D) Dry weight. Each data point represents the mean of three replications. Error bars represent standard errors. Bars with different letters are significantly different from each other at p<0.05.
The chlorophyll content of F. arundinacea showed the lowest value in 100,000 mg L-1 treatment, and 50,000 and 100,000 mg L-1 treatments significantly decreased compared with the control, 6,250, and 12,500 mg L-1 treatments (Fig. 4A). For chlorophyll content of T. repens, shoot extract treatments were significantly reduced compared with control. However, there was no significant difference between the extract treatment groups (Fig. 4B).
Chrysophanic acid extracted from R. acetosella is known to have a high herbicidal effect on Poaceae and broadleaf plants including T. repens (Kang et al., 2011; Choi et al., 2010). Based on these previous studies, R. acetosella shoot extract was also effective on F. arundinacea and T. repens in the foliage treatment. However, despite the high concentration of 100,000 mg L-1, there was no significant difference in shoot length, fresh weight, and dry weight from other treatment groups except for the control, which is thought that the growth of the two plants progressed for more than 4 weeks, and the herbicidal effect decreased (Jang et al., 2010). However, there was no significant difference in root length, which is thought to be because natural herbicidal active substances are generally contact-type rather than transition-type (Copping & Duke, 2007).

Fig. 4.Chlorophyll content of F. arundinacea and T. repens with foliage treatment by the concentration of extract of R. acetosella shoot. (A) Chlorophyll content of F. arundinacea, (B) Chlorophyll content of T. repens. Each data point represents the mean of three replications. Error bars represent standard deviations. Bars with different letters are significantly different from each other at p<0.05.
Conclusion
As a result of the treatment of the shoot and root parts of R. acetosella, the shoot part was relatively effective on the germination and growth of F. arundinacea and T. repens. In the case of foliage treatment, shoot extract was effective in shoot length, fresh weight, and dry weight of F. arundinacea and T. repens.
Acknowledgements
This study was supported by the joint research project from the Rural Development Administration, Republic of Korea (Project number: PJ015026022022).
Authors Information
Yosep Kang, Department of Applied Biosciences, Kyungpook National University, Master student
Ho-Jun Gam, Department of Applied Biosciences, Kyungpook National University, Master student
Eun-Jung Park, Department of Applied Biosciences, Kyungpook National University, Researcher
Bo-Ram Choi, National Institute of Animal Science, RDA, Researcher
Ki-Yong Kim, National Institute of Animal Science, RDA, Researcher
Sang-Mo Kang, Department of Applied Biosciences, Kyungpook National University, Doctor of Philosophy
In-Jung Lee, http://orcid.org/0000-0001-7154-4820